Method for semisynthesis of Docetaxel and intermediate of Docetaxel
A technology for docetaxel and intermediates, applied in the field of pharmaceutical chemical synthesis, can solve the problems of 10-DABⅢ raw material waste, easy reaction to generate other impurities, and loss of docetaxel products, so as to achieve less impurity generation and simple and easy operation Effect of control and less by-products
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
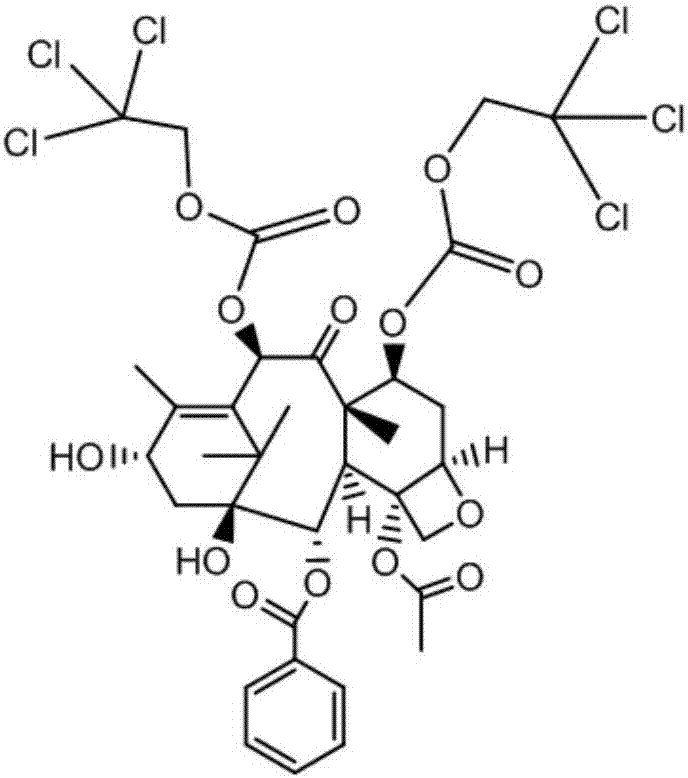
[0043] (1) Weigh the raw material of 10-DABⅢ, add dry pyridine and stir to dissolve, then add 2,2,2-trichloroethyl chloroformate dropwise under the protection of nitrogen, the dropping time is kept at 20min, and the dropping temperature is controlled at 0 ℃, after the dropwise addition is completed, control the reaction temperature to 10°C, stop the reaction when the 7-hydroxyl protected by-product of 10-DABⅢ in the reaction solution is 5%, quench the reaction by adding water dropwise, and then neutralize pyridine with concentrated hydrochloric acid , extracted with dichloromethane, concentrated and dried to obtain the crude intermediate Ⅰ.
[0044] Among them, the mass ratio of 10-DABⅢ raw material, dry pyridine, and 2,2,2-trichloroethyl chloroformate is 1:3:0.6.
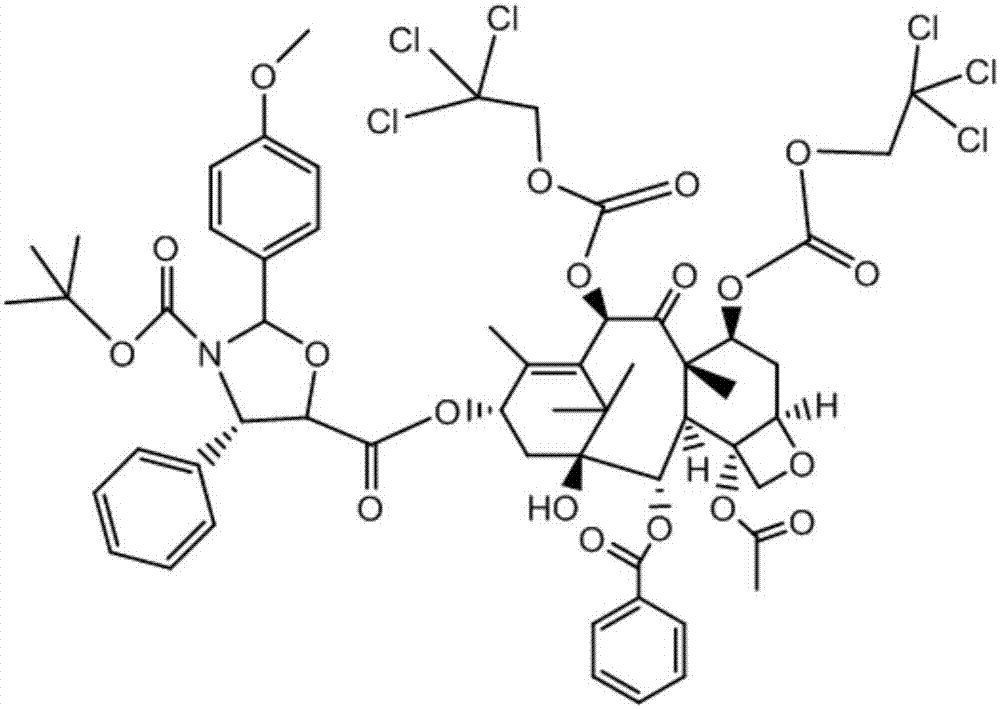
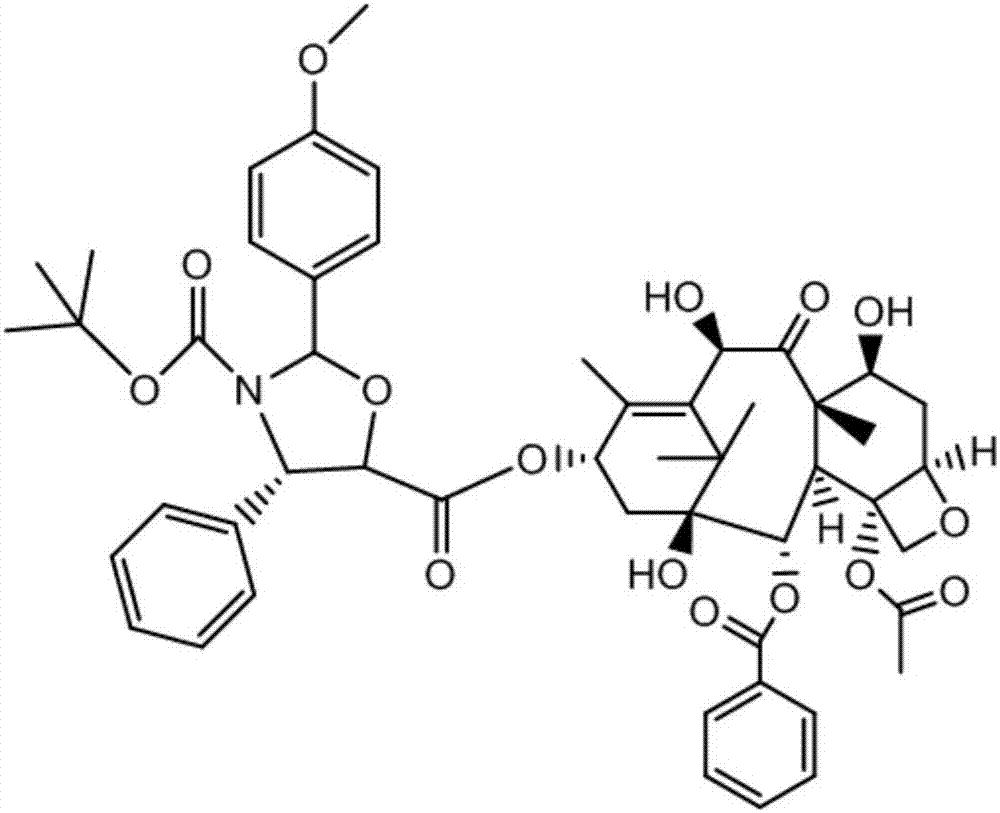
[0045] (2) Weigh the crude product of intermediate I, dissolve it with an organic solvent, filter, and then separate and collect the eluate containing intermediate I and the 7-position hydroxyl protection by-produc...
Embodiment 2
[0057] (1) Weigh the 10-DABⅢ raw material, add dry pyridine and stir to dissolve, then add 2,2,2-trichloroethyl chloroformate dropwise under the protection of nitrogen, the dropping time is kept at 40min, and the dropping temperature is controlled at 10 ℃, after the dropwise addition is completed, control the reaction temperature to 35°C, stop the reaction when the 7-hydroxyl protected by-product of 10-DABⅢ in the reaction liquid is 10%, add water dropwise to quench the reaction, and then neutralize pyridine with concentrated hydrochloric acid , extracted with dichloromethane, concentrated and dried to obtain the crude intermediate Ⅰ.
[0058] Among them, the mass ratio of 10-DABⅢ raw material, dry pyridine, and 2,2,2-trichloroethyl chloroformate is 1:8:2.
[0059] (2) Weigh the crude product of intermediate I, dissolve it in an organic solvent, filter it, and then separate and collect the eluent containing intermediate I and the 7-position hydroxyl protection by-product throu...
Embodiment 3
[0071] (1) Weigh the 10-DABⅢ raw material, add dry pyridine and stir to dissolve, then add 2,2,2-trichloroethyl chloroformate dropwise under the protection of nitrogen, the dropping time is kept at 30min, and the dropping temperature is controlled at 5 ℃, after the dropwise addition is completed, control the reaction temperature to 20°C, stop the reaction when the 7-position hydroxyl protection by-product of 10-DABⅢ in the reaction solution is 8%, quench the reaction by adding water dropwise, and then neutralize the pyridine with concentrated hydrochloric acid , extracted with dichloromethane, concentrated and dried to obtain the crude intermediate Ⅰ.
[0072] Among them, the mass ratio of 10-DABⅢ raw material, dry pyridine, and 2,2,2-trichloroethyl chloroformate is 1:5:1.3.
[0073] (2) Weigh the crude product of intermediate I, dissolve it with an organic solvent, filter, and then separate and collect the eluate containing intermediate I and the 7-position hydroxyl protectio...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com