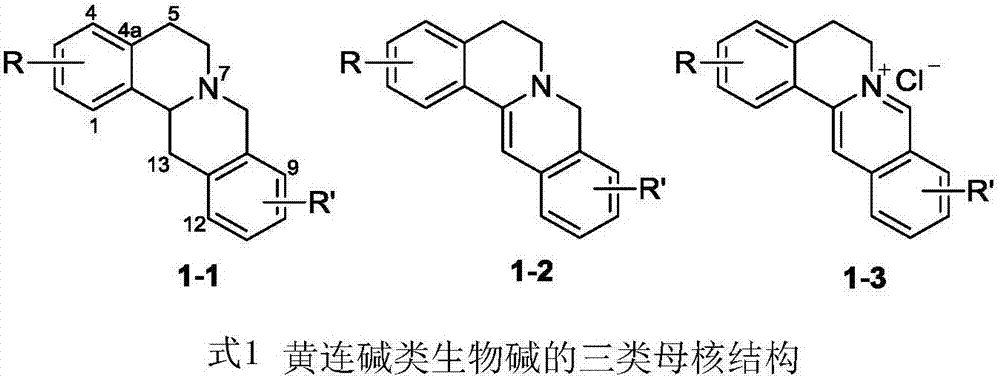
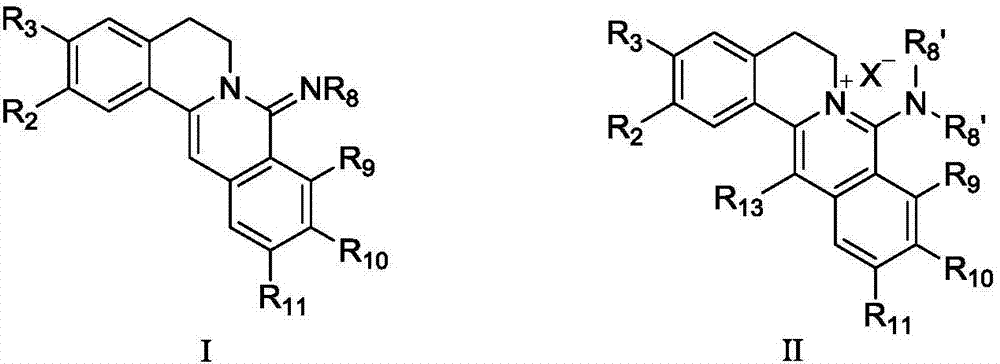
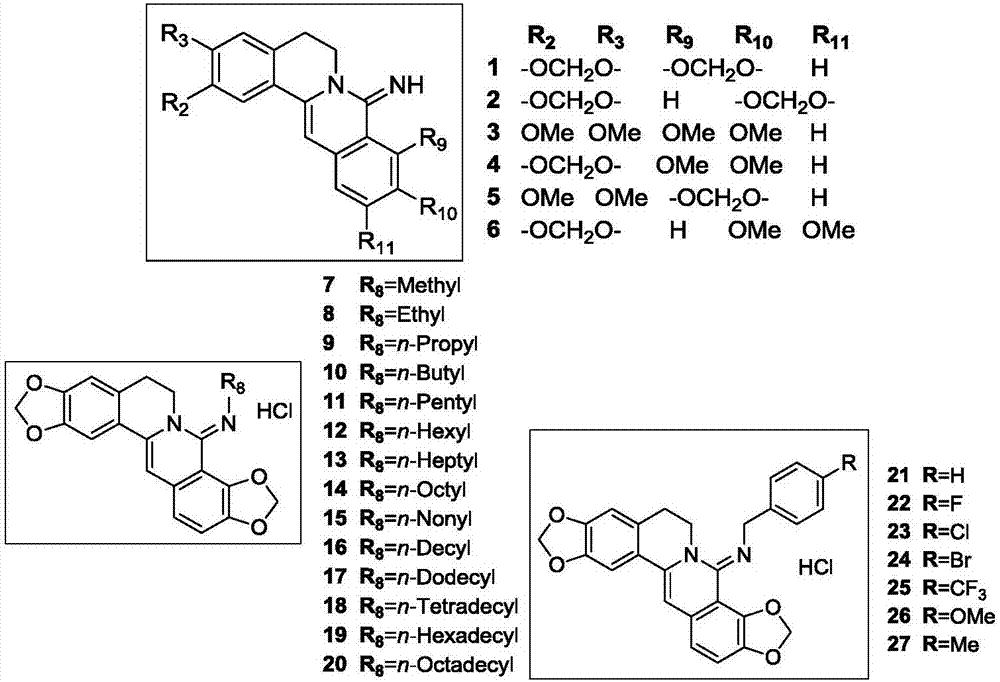
Coptisine derivatives, as well as preparation method, medicinal composition and anti-tumor application thereof
A technology of coptisine and its derivatives, which is applied in the field of coptisine derivatives, its preparation, pharmaceutical composition and anti-tumor application, and can solve the problems of poor anti-tumor activity and specificity, low bioavailability, and high toxicity to human body
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment (1
[0052] Example (1) Preparation process and structural identification data of compound 1
[0053] Weigh KOH (5N, 200ml) in the reaction flask, add K at room temperature 3 [Fe(CN) 6 ] (46.27g, 140.54mmol), heated to 45°C and stirred to dissolve, added in batches the substrate coptisine chloride quaternary ammonium salt (10g, 28.10mmol), then heated to reflux for 8h, TLC monitored the reaction was complete, suction filtered, washed to neutral. A light yellow crude product was obtained, which was purified by silica gel column chromatography [v / v=100:1 (chloroform / methanol)] to obtain 5.75 g of a yellow solid with a yield of 61%, namely the intermediate 8-dihydrocoptisine oxide; 1 H NMR (DMSO-d 6 ,400MHz)δ 2.85(t,J=6.0Hz,2H,Ar CH 2 CH 2 N), 4.08(t, J=6.0Hz, 2H, ArCH 2 CH 2 N),6.06(s,2H,OCH 2 O),6.18(s,2H,OCH 2 O),6.91(s,1H,Ar- H ),7.11(s,1H,Ar- H ), 7.14 (d, J=8.4Hz, 1H, Ar- H ), 7.33 (d, J=8.4Hz, 1H, Ar- H ),7.46(s,1H,Ar- H ). 13 C NMR (DMSO-d 6 ,150MHz)δ 27.55...
Embodiment
[0054] Example (2) Preparation process and structural identification data of compound 7
[0055] Weigh 8-chlorocoptisine chloride quaternary ammonium salt (200mg, 0.51mmol) in a reaction flask, add toluene (20ml), slowly add methylamine (40.4μl, 1.02mmol) under the protection of argon, and react at room temperature for 2h, TLC monitored the completion of the reaction. Add 5ml of anhydrous diethyl ether, pass through dry HCl gas until no excessive precipitation occurs, filter with suction, add water (30ml) and a small amount of methanol to the filter cake, and use CHCl respectively 3 (50ml) extraction twice; take chloroform solution, extract once with saturated brine, anhydrous MgSO 4 Dry, filter with suction, and evaporate the solvent to dryness under reduced pressure to obtain a light yellow crude product, which is purified by silica gel column chromatography [v / v=20:1 (chloroform / methanol)] to obtain compound 7, a yellow solid of 73.76mg, with a yield of 37.4%; 1 H NMR (DM...
Embodiment (7
[0064] Example (7) preparation process and structural identification data of compound 12
[0065]Weigh 8-chlorocoptisine chloride quaternary ammonium salt (200mg, 0.51mmol) in a reaction flask, add toluene (20ml), slowly add hexylamine (134.7μl, 1.02mmol) under the protection of argon, and react at room temperature for 2h, TLC monitored the completion of the reaction. Add 5ml of anhydrous diethyl ether, pass through dry HCl gas until no excessive precipitation occurs, filter with suction, add water (30ml) and a small amount of methanol to the filter cake, and use CHCl respectively 3 (50ml) extraction twice; take chloroform solution, extract once with saturated brine, anhydrous MgSO 4 dry. Suction filtration and evaporation of the solvent under reduced pressure gave a light yellow crude product, which was purified by silica gel column chromatography [v / v=20:1 (chloroform / methanol)] to obtain 187 mg of compound 12 as a yellow solid with a yield of 80.2%; 1 H NMR (DMSO-d 6 ,4...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com