A crystal form of dirithromycin compound and its crystal preparation method
A dirithromycin compound technology, applied in the field of dirithromycin compound crystal form and its crystallization preparation, can solve the problem of unsatisfactory solubility, crystal form and fluidity, large amount of mixed organic solvents, and difficulty in solvent recovery To achieve the effect of facilitating the implementation of large-scale industrialization, improving bioavailability, and shortening the experimental cycle
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
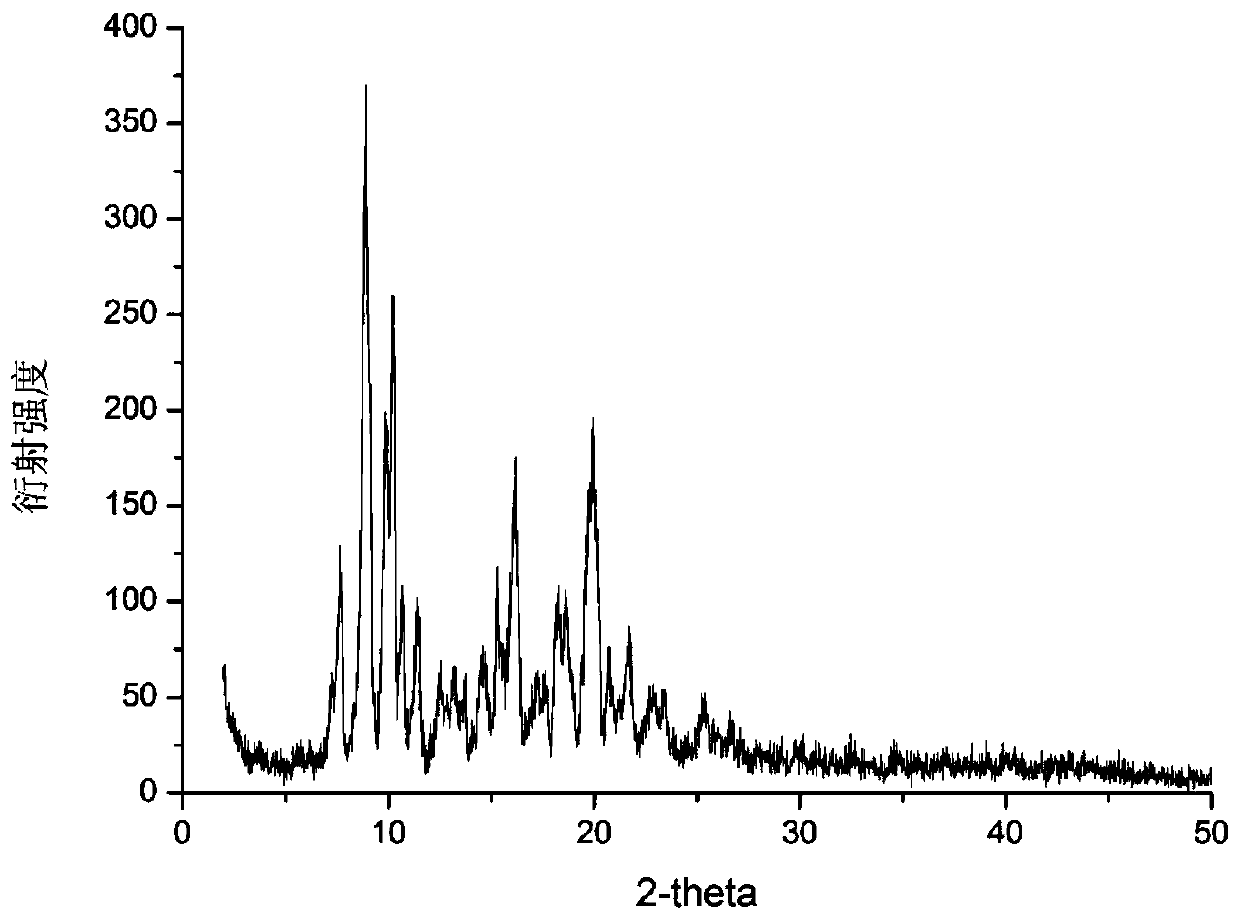
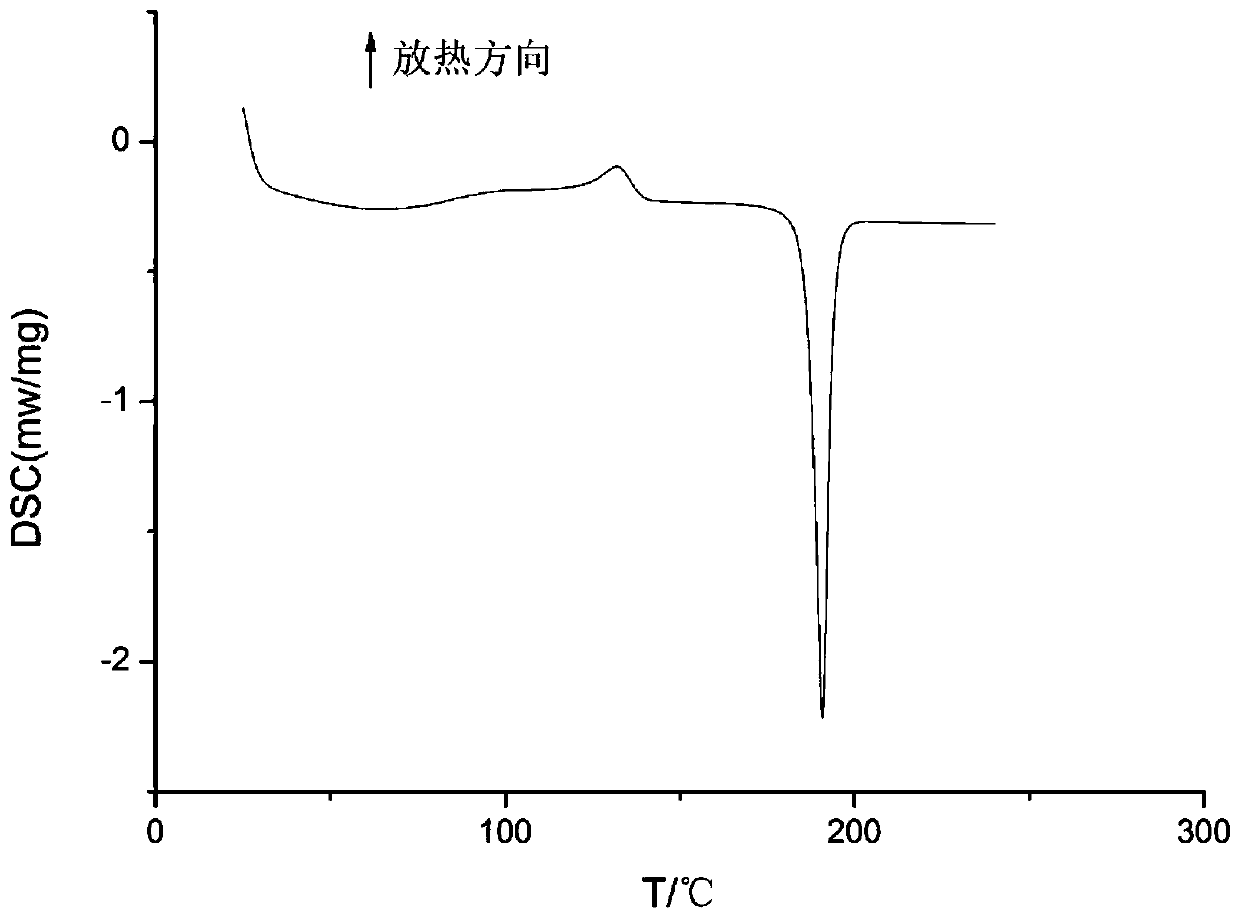
[0029] At room temperature, add 1.05 g of dierythromycin solid to 30 ml of acetonitrile, and form a suspension at a stirring rate of 250r / min. The temperature is raised to 50°C, and the solid is completely dissolved. Continue to stir the solution, and then place it in an ultrasonic system , Then cool the mixture from 50°C to 0°C at a cooling rate of 1.5°C / min, turn on the ultrasonic generator 5 minutes after the start of the temperature drop, and open the device for 10 minutes; continue to stir for 5 hours after the end of the temperature drop, and the stirring rate will be maintained during the experiment No change; suction filtration, drying the obtained product at 77° C. under vacuum conditions for 24 hours to obtain a new crystal form of dierythromycin. The X-ray powder diffraction pattern of the new crystal form product is as follows figure 1 As shown, it is at the diffraction angle 2θ=5.84, 7.23, 7.70, 8.90, 9.93, 10.17, 10.67, 11.42, 13.09, 14.70, 15.33, 16.16, 17.59, 18....
Embodiment 2
[0031] Add 1.50g of dry dierythromycin solid to 30ml of acetonitrile, form a suspension at a stirring rate of 450r / min, heat to 74°C, the solids are completely dissolved, continue to stir the solution, and then cool down at a rate of 0.4°C / min Cool the mixture from 74°C to 5°C, turn on the ultrasonic generator 70min after the start of the temperature drop, and turn on the ultrasonic generator for 15 minutes. After the temperature drop, the temperature will be kept constant, and the crystal will be stirred for 3h. The stirring rate will remain unchanged during the experiment; To obtain a crystal slurry, the obtained product is dried at 95° C. under vacuum conditions for 24 hours to a constant weight to obtain a new crystal form of dierythromycin. The X-ray powder diffraction pattern of the product is at diffraction angle 2θ = 5.76, 7.23, 7.70, 8.89, 9.94, 10.19, 10.66, 11.38, 13.14, 14.65, 15.42, 16.20, 17.64, 18.19, 18.88, 19.85, 20.60, 21.51, 22.76 , There is a characteristic ...
Embodiment 3
[0033] At room temperature, add 2.53g of dierythromycin solid to 60ml of acetonitrile, and form a suspension at a stirring rate of 400r / min. The temperature is increased to 74°C, the solid is completely dissolved, the solution is continuously stirred, and then cooled at 1.0°C / min Cool the mixture from 74°C to 0°C at a rate. Turn on the ultrasonic generator 45min after the start of the cooling. The open time is 13min. After the cooling process is over, the temperature is kept constant and the crystal is stirred for 4h. The stirring rate remains unchanged during the experiment; The crystal slurry is suction filtered, and the obtained product is dried at 85° C. under vacuum conditions for 24 hours to constant weight to obtain a new crystal form of dierythromycin. The X-ray powder diffraction pattern of the product is at diffraction angle 2θ = 5.84, 7.33, 7.72, 8.94, 9.99, 10.09, 10.66, 11.37, 13.14, 14.65, 15.36, 16.02, 17.67, 18.20, 18.80, 19.82, 20.62, 21.51, 22.72 , There is a ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com