Cobalt-based catalyst for preparing hydrogen through acetic acid autothermal reforming and preparation method
A cobalt-based catalyst, autothermal reforming technology, applied in the direction of chemical instruments and methods, metal/metal oxide/metal hydroxide catalyst, heterogeneous catalyst chemical elements, etc., can solve the problem of easy oxidation of active component cobalt and Solve problems such as sintering, easy structure change, and catalyst deactivation, and achieve the effects of increasing reducibility, inhibiting migration, and high hydrogen production rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 2
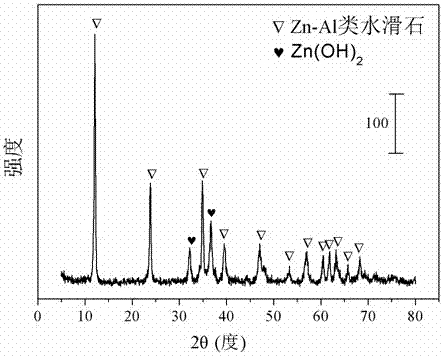
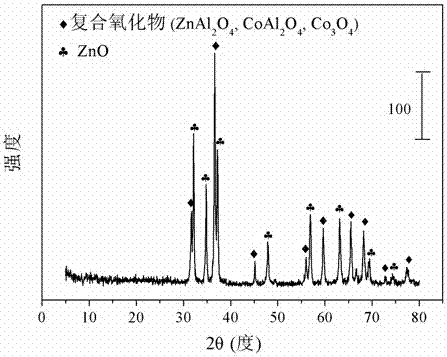
[0027] Weigh 5.4622 grams of Co(NO 3 ) 3 .6H 2 O, 27.9172 g Zn(NO 3 ) 3 .6H 2 O and 7.0406 g Al(NO 3 ) 3 .9H 2 O, add 131 mL of deionized water and mix to form solution #1. Weigh 12.012 grams of sodium hydroxide and 1.9893 grams of sodium carbonate and add 319 milliliters of deionized water to form solution #2. Mix solution #1 and solution #2 at pH 10.5 + Within the range of 0.5, the co-precipitation operation was carried out in a water bath of 78 degrees Celsius, and the temperature was maintained for 24 hours with stirring and aging. The precipitate was filtered and washed three times with deionized water, and dried in an oven at 105 degrees Celsius for 12 hours to obtain a hydrotalcite-like precursor, whose typical structure is shown in the attached figure 1 shown. The precursor was calcined at 700 degrees Celsius for 4 hours to obtain the catalyst CDUT-ZC6A, whose typical structure is as follows figure 2 shown. After nitrogen adsorption / desorption experiments...
Embodiment 1
[0030] Weigh 5.5134 grams of Co(NO 3 ) 3 .6H 2 O, 22.5415 grams of Zn (NO 3 ) 3 .6H 2 O and 17.7653 g Al(NO 3 ) 3 .9H 2 O, add 142 mL of deionized water and mix to form solution #1. Weigh 21.2162 g of sodium hydroxide and 3.5136 g of sodium carbonate and add 564 ml of deionized water to form solution #2. Mix solution #1 and solution #2 at pH 10.5 + Within the range of 0.5, the co-precipitation operation was carried out in a water bath of 78 degrees Celsius, and the temperature was maintained for 24 hours with stirring and aging. The precipitate was filtered and washed three times with deionized water, and dried in an oven at 105 degrees Celsius for 12 hours to obtain a hydrotalcite-like precursor, whose typical structure is shown in the attached figure 1 shown. The precursor is calcined at 700 degrees Celsius for 4 hours to obtain the catalyst CDUT-ZC2A, whose typical structure is as follows figure 2 shown. After nitrogen adsorption / desorption experiments, the re...
Embodiment 2
[0033] Weigh 5.4898 g Co(NO 3 ) 3 .6H 2 O, 24.9974 g Zn(NO 3 ) 3 .6H 2 O and 12.8658 g Al(NO 3 ) 3 .9H 2 O, add 137 mL of deionized water and mix to form solution #1. Weigh 17.0113 g of sodium hydroxide and 2.8172 g of sodium carbonate and add 452 ml of deionized water to form solution #2. Mix solution #1 and solution #2 at pH 10.5 + Within the range of 0.5, the co-precipitation operation was carried out in a water bath of 78 degrees Celsius, and the temperature was maintained for 24 hours with stirring and aging. The precipitate was filtered and washed three times with deionized water, and dried in an oven at 105 degrees Celsius for 12 hours to obtain a hydrotalcite-like precursor, whose typical structure is shown in the attached figure 1 shown. The precursor was calcined at 700 degrees Celsius for 4 hours to obtain the catalyst CDUT-ZC3A, whose typical structure is as follows figure 2 shown. After nitrogen adsorption / desorption experiments, the results show tha...
PUM
| Property | Measurement | Unit |
|---|---|---|
| specific surface area | aaaaa | aaaaa |
| specific surface area | aaaaa | aaaaa |
| specific surface area | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com