An immunoassay for multiple pde-5 inhibitor drugs
A technology of PDE-5 and inhibitors, applied in the field of immunoassay, to achieve broad application prospects, rapid detection, and better sensitivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0048] The preparation of embodiment 1 immunogen / coating original
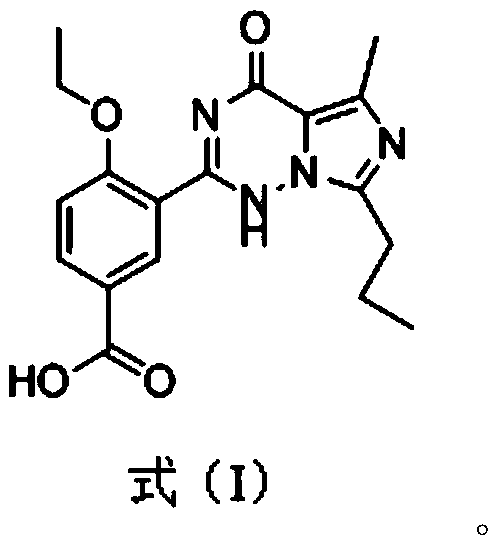
[0049] 1. The present invention has synthesized a novel small molecule compound by analyzing the spatial structure of several PDE-5 inhibitor drugs, as shown in formula (I), it can be used as a hapten for preparing artificial antigens and antibodies for detecting multiple A PDE-5 inhibitor drug.
[0050]
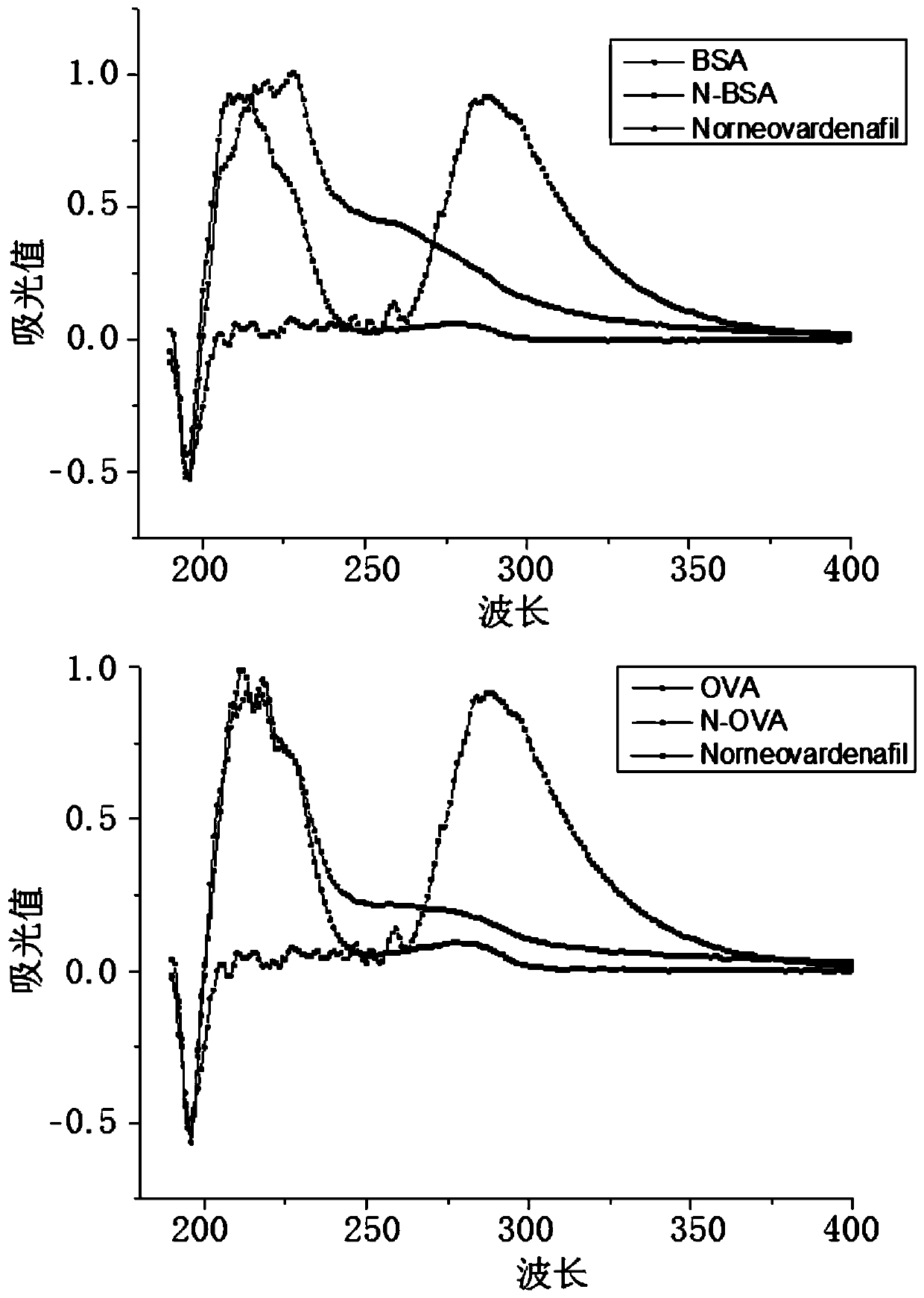
[0051] 2. In the preparation method of the immunogen used in the present invention and the coating original, the difference lies in the use type of the carrier protein, the immunogen carrier protein mainly adopts bovine serum albumin (BSA), and the described coating former carrier protein mainly adopts egg white Protein (OVA), the coupling method used is active ester method. Take the preparation method of the following immunogen as an example.
[0052] (1) Active ester method: Dissolve 5 mg (0.015 mol) of the hapten Norneovardenafil in 0.3 mL of DMF, add 4.78 mg of DCC (0.023 mol), 2.65 mg of NHS (0.023...
Embodiment 2
[0058] Example 2 Antibody Preparation and Identification
[0059] The prepared immunogen was evenly emulsified with an equal amount of immune adjuvant (incomplete Freund's adjuvant was used for the first immunization, and incomplete Freund's adjuvant was used for subsequent booster immunizations), and animals were immunized. New Zealand white rabbits weighing 2.5 to 3 kg were immunized by subcutaneous injections on the back, subcutaneous injections in various parts, leg muscles, and ear veins. The second immunization was performed 4 weeks later, and the immunization was added every 3 weeks thereafter. One week after the fourth booster immunization, blood was collected from the ear vein, and the serum titer was determined by indirect competitive ELISA. When the titer no longer rises, the ear vein is used to boost the immunization. One week later, blood was collected from the heart, bathed in water for 0.5-1 hour, centrifuged at 10,000°C for 15 minutes at 4°C, and the supernata...
Embodiment 3
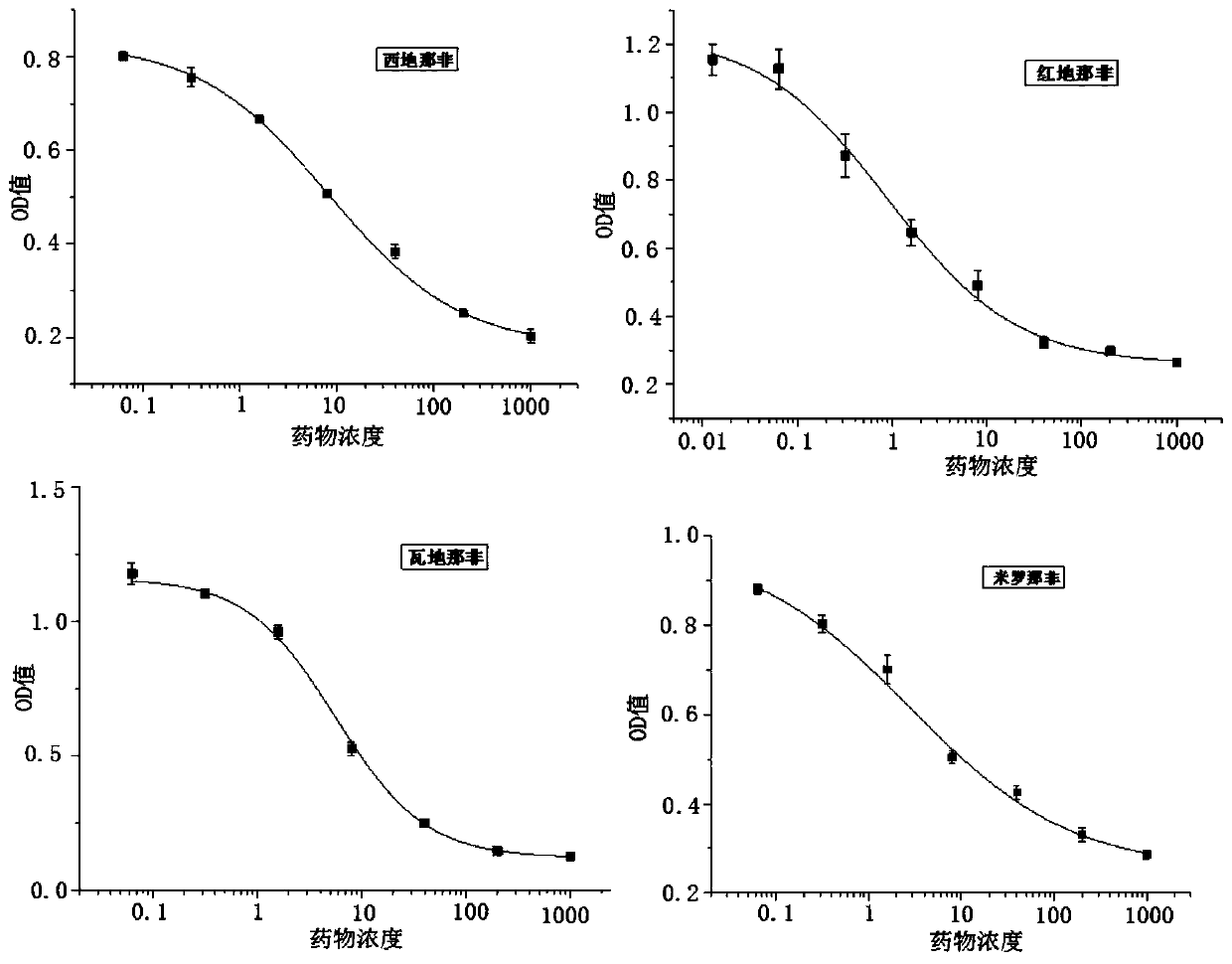
[0061] The specificity and sensitivity of embodiment 3 antibody
[0062] 1. According to the above effect, use antiserum to draw enzyme-linked immunoassay (ELISA) standard curve; use phosphate Tween buffer (PBST, 0.1mol / L, pH=7.4) as the diluent of all samples: 50 μL of serial concentrations Drug standards and 50 μL of multispecific antibodies with appropriate dilution times were added to a 96-well microplate plate, and the absorbance (OD) was measured by a microplate analyzer after the competition reaction. Take the OD value as the vertical axis, and the corresponding logarithm value of the standard substance concentration as the horizontal axis, and use the four parameters of origin9.1 software to perform curve fitting on the function: y=(A-D) / [1+(X / C)B]+D
[0063] Among them, A and D represent the minimum and maximum absorbance values (OD) of the drug concentration, respectively, and C is the midpoint concentration. When the standard concentration is equal to C, the OD va...
PUM
| Property | Measurement | Unit |
|---|---|---|
| recovery rate | aaaaa | aaaaa |
| recovery rate | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com