Method for preparing high-quality levofloxacin hydrochloride
A high-quality technology of levofloxacin hydrochloride, applied in organic chemistry and other directions, can solve the problems of increased post-processing workload and three wastes, poor environmental protection and economy, no specific introduction, etc., to reduce environmental pollution, industrial raw material consumption, and low production costs. , the effect of simplifying the process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
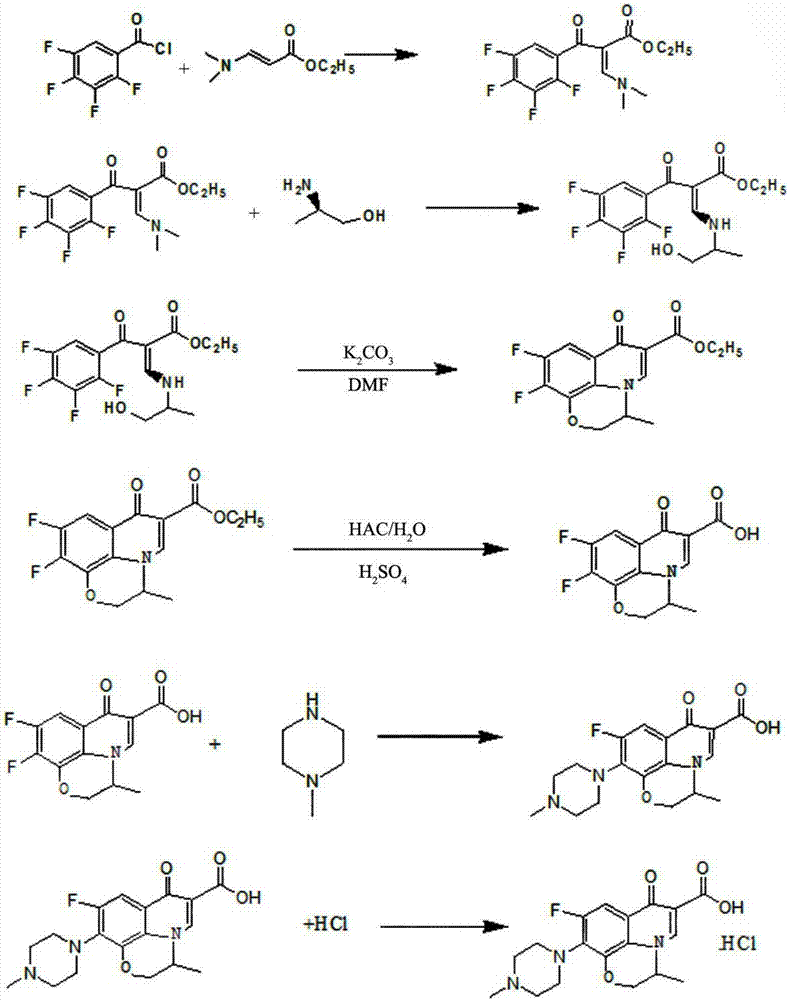
[0044] Such as figure 1 The synthetic method of shown levofloxacin hydrochloride comprises the following steps:
[0045] (1) Preparation of 3-(2-hydroxy-1-methyl-ethylamino)-2-(2,3,4,5-tetrafluorobenzoyl)-ethyl acrylate
[0046] Add 29.2g of N,N ethyl dimethylaminoacrylate, 22.7g of triethylamine and 340ml of toluene into a three-necked flask, heat to 50°C under stirring, slowly add a solution of 42.5g of tetrafluorobenzoyl chloride / toluene, dropwise Add for 1.5 hours, keep warm for 3 hours after dropping, convert 97%, cool, filter about 24.2g of triethylamine hydrochloride, wash the filter cake of triethylamine hydrochloride with a small amount of toluene, heat the filtrate to 50°C, add 15g of triethylamine hydrochloride dropwise L-2-Aminopropanol, dripped in half an hour, raised the temperature to 90°C, kept it warm for 1 hour, the conversion was 98%, cooled, washed twice with water, the toluene phase was separated, spin-dried and dehydrated, diluted with DMF to 250ml, liquid...
Embodiment 2
[0053] Such as figure 1 The synthetic method of shown levofloxacin hydrochloride comprises the following steps:
[0054] (1) Preparation of 3-(2-hydroxy-1-methyl-ethylamino)-2-(2,3,4,5-tetrafluorobenzoyl)-ethyl acrylate
[0055] Add 29.2g of N,N ethyl dimethylaminoacrylate, 20.2g of triethylamine and 340ml of toluene into a three-necked flask, heat it to 40°C under stirring, slowly add a solution of 46.8g of tetrafluorobenzoyl chloride / toluene dropwise, drop Add for 1 hour, keep warm for 2 hours after dropping, convert 96%, cool, filter about 24.8g of triethylamine hydrochloride, wash the filter cake of triethylamine hydrochloride with a small amount of toluene, heat the filtrate to 40°C, add 18.4g dropwise The L-2-aminopropanol was dropped in half an hour, heated to 90°C, kept warm for 0.5 hours, converted 98%, cooled, washed three times with water, separated into toluene phases, spin-dried and dehydrated, diluted with DMF to 250ml, liquid The phase purity is 99.22%.
[00...
Embodiment 3
[0062] Such as figure 1 The synthetic method of shown levofloxacin hydrochloride comprises the following steps:
[0063] (1) Preparation of 3-(2-hydroxy-1-methyl-ethylamino)-2-(2,3,4,5-tetrafluorobenzoyl)-ethyl acrylate
[0064] Add 29.2g of N,N ethyl dimethylaminoacrylate, 24.3g of triethylamine and 340ml of toluene into a three-necked flask, heat to 60°C under stirring, slowly add 45g of tetrafluorobenzoyl chloride / toluene solution dropwise, dropwise After 2 hours, keep warm for 4 hours after dripping, convert 97%, cool, filter about 25.2g of triethylamine hydrochloride, wash the filter cake of triethylamine hydrochloride with a small amount of toluene, heat up the filtrate to 60°C, add dropwise 17g of L -2-Aminopropanol, drop it in half an hour, raise the temperature to 90°C, keep it warm for 1.5 hours, the conversion is 98%, cool, wash twice with water, separate the toluene phase, spin dry and dehydrate, add DMF to dilute to 250ml, liquid phase The purity is 99.41%.
[...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com