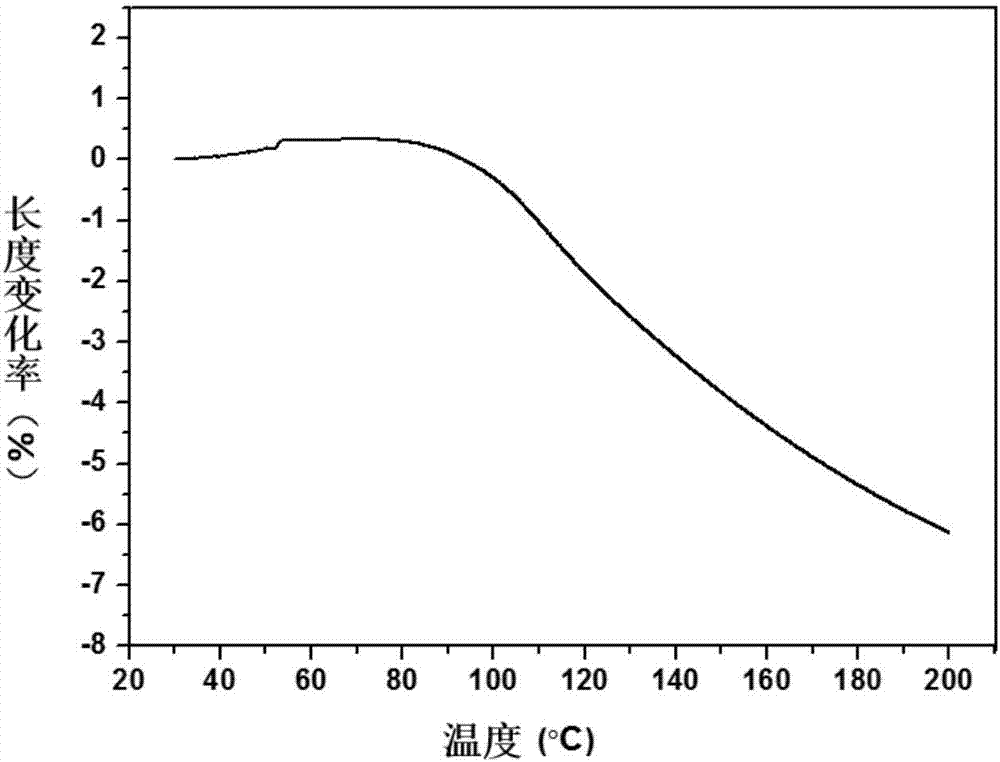
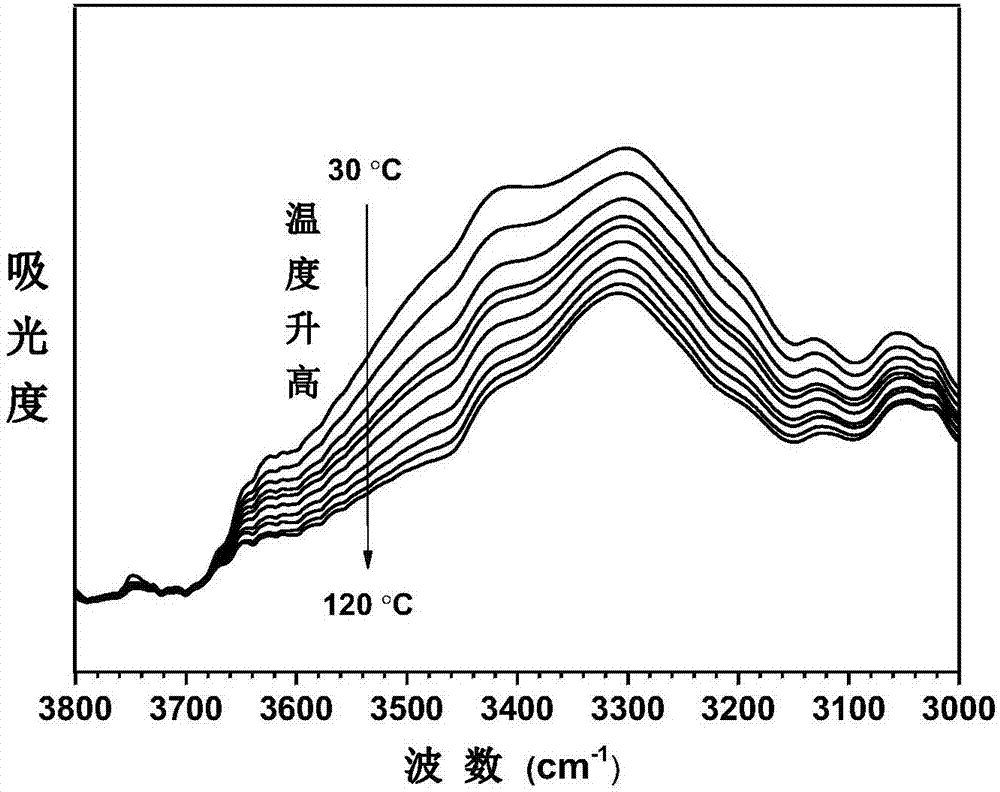
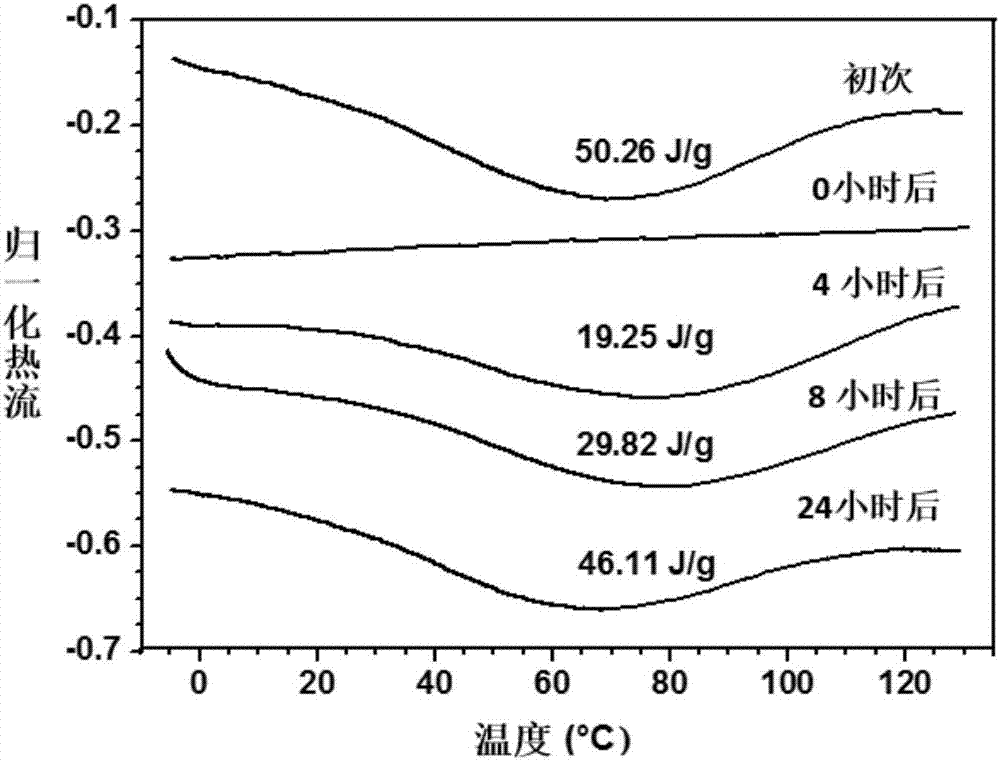
Thermal shrinkage-reversible polyarylamide with dibenzo 8-membered ring (DBCOD) structure containing main chain and preparation method thereof
A technology of polyarylamide and eight-membered ring, which is applied in the field of reversible heat-shrinkable polyarylamide containing dibenzo eight-membered ring structure in the main chain and its preparation field, achieving the effect of simple and feasible synthetic route and correct product structure
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Example 1, 5,6,11,12-tetrahydrodibenzo[a,e][8]annulene-2,9-dicarboxylic acid preparation of
[0034] Step 1: Add methyltriphenylphosphine iodide (21.0g, 52mmol) into a pre-dried 250mL Shrek tube, replace nitrogen three times, then add 40mL ultra-dry tetrahydrofuran through a syringe to form a milky white suspension. At 20°C, add potassium tert-butoxide (5.83g, 52mmol) and continue stirring for 15-30min, then slowly add dibenzocycloheptanone (8.33g, 40.0mmol) through a syringe, and continue stirring at 40°C Reaction 10 ~ 15h. After removing the ylide produced by the reaction through silica gel, 7.67 g of white powder (compound 1) was obtained after column chromatography purification (dichloromethane:n-hexane=1:10), with a yield of 93%. 1 H NMR (400MHz, Acetone-d 5 )δ7.34(d,J=7.0Hz,2H),7.25–7.11(m,6H),5.40(s,2H),3.11(s,4H). 13 C NMR (101MHz, Acetone-d 5 )δ152.44, 141.26, 138.51, 129.15, 128.15, 127.99, 126.43, 116.91, 33.24. MS (ESI) m / z [M-H] + 205.2.
[0035] St...
Embodiment 2
[0039] Example 2, 5,6,11,12-tetrahydrodibenzo[a,e][8]annulene-2,8-dicarboxylic acid preparation of
[0040] Step 1 is the same as Step 1 in Example 1.
[0041] Step 2, add 30mL of anhydrous methanol to a 100mL three-necked reaction flask, heat to 80°C, add silver nitrate (1.43g, 8.4mmol), stir well to dissolve; continue to add iodine (1.07g, 4.2mmol) and compound 1 (814mg, 4mmol), immediately add 30mL of dioxane, keep the temperature under reflux reaction for 24h; distill off the solvent under reduced pressure, add 50mL of dichloromethane and 50mL of water, separate the organic phase, and wash three times with anhydrous sulfuric acid The organic phase was dried over sodium, concentrated under reduced pressure to obtain a yellow oily solid, and purified by column chromatography (dichloromethane: n-hexane = 1:8) to obtain 433 mg of white powder (compound 2), with a yield of 52%. 1 H NMR (400MHz, Acetone-d 5 )δ7.39–6.95(m,6H),4.18(s,2H),3.34(qd,J=8.8,2.2Hz,4H). 13 C NMR (101...
Embodiment 3
[0045] Embodiment 3, the synthesis of polyarylamide A
[0046] Polyarylamide was prepared by Yamazaki-Higashi phosphorylation polycondensation reaction technique. Add 492.6mg (1.5mmol) of 5,6,11,12-tetrahydrodibenzo[a,e][8]annulene-2,9-dicarboxylic acid to a 25mL Shrek tube, 3,4'-di Aminodiphenyl ether 300.3mg (1.5mmol), 1.0mL triphenyl phosphite, 0.6mL pyridine, 300.0mg calcium chloride and 3.0mL ultra-dry N-methyl-2-pyrrolidone (NMP), freeze-thaw and replace nitrogen After three times, it was placed in an oil bath at 120°C for 10 hours. After the reaction liquid was lowered to room temperature, it was slowly poured into 200 mL of methanol to obtain a fibrous precipitate. The product was further washed with methanol and hot water, and dried in vacuum for 24 hours to obtain polyarylamide.
PUM
| Property | Measurement | Unit |
|---|---|---|
| elongation | aaaaa | aaaaa |
| shrinkage | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com