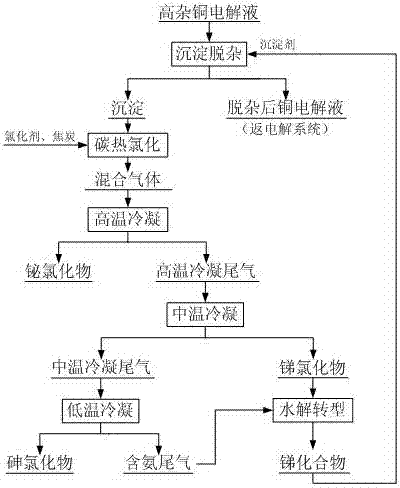
Method for carrying out precipitate impurity removal on copper electrolyte and carrying out chlorination regeneration on precipitant
A technology of copper electrolyte and precipitant, which is applied in the field of bismuth impurity removal and comprehensive recovery, arsenic and antimony in copper electrolyte, can solve the problems of long process flow, low impurity removal efficiency, side effects of electrolyte, etc. Short, high removal rate, simple operation effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019] to 1 m 3 Add 15 kg of antimony trioxide to the copper electrolyte, react at a temperature of 85 °C for 1.0 hour, and filter to obtain a copper electrolyte of 0.99 m 3 and 24.46 kg of precipitation containing arsenic, antimony and bismuth, the removal rates of arsenic, antimony and bismuth in the copper electrolyte were 68.89%, 64.22% and 92.75%, respectively, and the precipitation reaction had a great influence on the copper and acid content in the copper electrolyte. The results of precipitation and impurity removal are as follows.
[0020] element
[0021] Mix the precipitate containing arsenic, antimony and bismuth with ammonium chloride and coke at a mass ratio of 10:20:1, and carry out carbothermal chlorination at a temperature of 800°C for 3.0 hours to obtain a mixture of chlorides containing arsenic, antimony and bismuth Gas 22.69 Nm 3 ; The mixed gas passed through a high-temperature condenser at 380°C and condensed for 2.0 hours to obtain 1.55 kg of...
Embodiment 2
[0023] to 1 m 3 Add 20 kg of antimony trioxide to the copper electrolyte, react at a temperature of 75 °C for 1.5 hours, and filter to obtain a copper electrolyte of 0.99 m 3 and 34.96 kg of precipitation containing arsenic, antimony and bismuth, the removal rates of arsenic, antimony and bismuth in the copper electrolyte were 72.56%, 85.71% and 94.25%, respectively, and the precipitation reaction had a great influence on the copper and acid content in the copper electrolyte. The results of precipitation and impurity removal are as follows.
[0024] element
[0025] Mix the precipitate containing arsenic, antimony and bismuth with ammonium chloride, ferrous chloride and coke at a mass ratio of 10:15:5:2, and carry out carbothermal chlorination at a temperature of 900°C for 2.5 hours to obtain arsenic, Mixed gas of antimony and bismuth chloride 24.58 Nm 3 ; The mixed gas was condensed for 1.5 hours through a high-temperature condenser at 350°C to obtain 3.00 kg of b...
Embodiment 3
[0027] to 1 m 3 Add 35 kg of antimony trioxide to the copper electrolyte, react at a temperature of 70 °C for 2.5 hours, and filter to obtain a copper electrolyte of 0.98 m 3and 57.73 kg of precipitation containing arsenic, antimony and bismuth, the removal rates of arsenic, antimony and bismuth in the copper electrolyte were 70.69%, 80.52% and 93.54%, respectively, and the precipitation reaction had a great influence on the copper and acid content in the copper electrolyte. The results of precipitation and impurity removal are as follows.
[0028] element
[0029] Mix the precipitate containing arsenic, antimony and bismuth with ammonium chloride, ferrous chloride, magnesium chloride and coke according to the mass ratio of 10:15:3:2:2, and carry out carbothermal chlorination at a temperature of 700°C for 4.0 hours, Obtain a mixed gas containing arsenic, antimony and bismuth chloride 39.75Nm 3 ; The mixed gas passed through a high-temperature condenser at 300°C and...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com