Method for measuring content of copper, cadmium, nickel and cobalt in zinc electrolyte simultaneously
A zinc electrolyte and content technology, applied in the field of analysis and testing, can solve the problems of simultaneous determination of difficult metal ions, real-time monitoring of unfavorable impurity ions, low sensitivity of spectrophotometry, etc., to achieve accurate and reliable measurement results, fast detection speed, and selective sex high effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
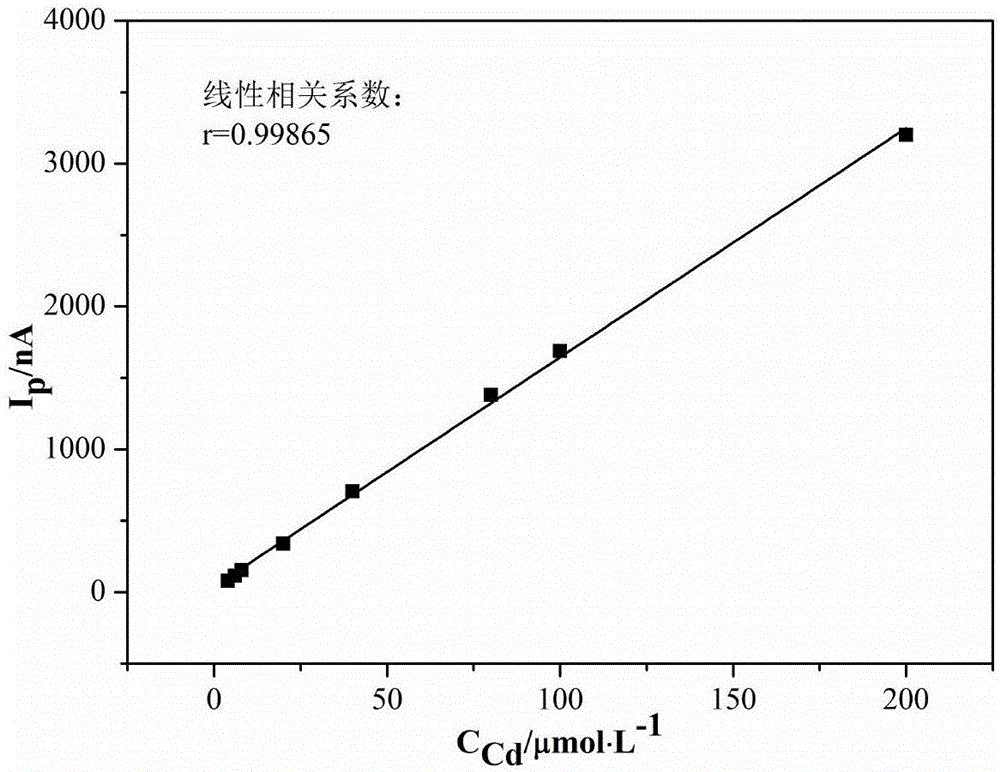
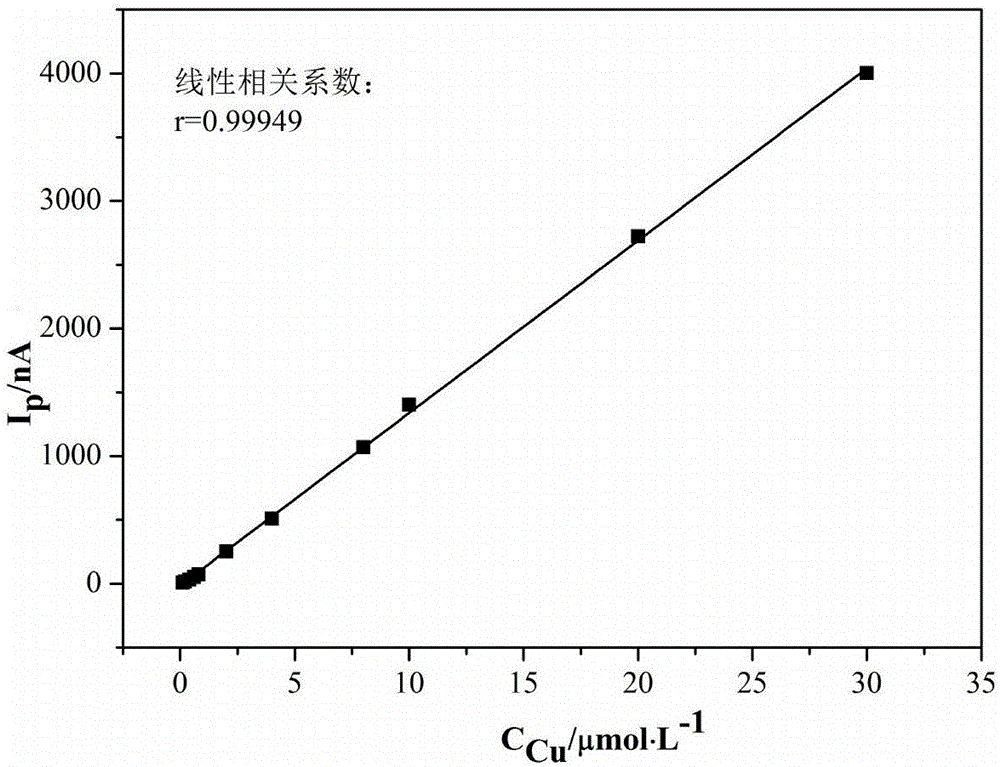
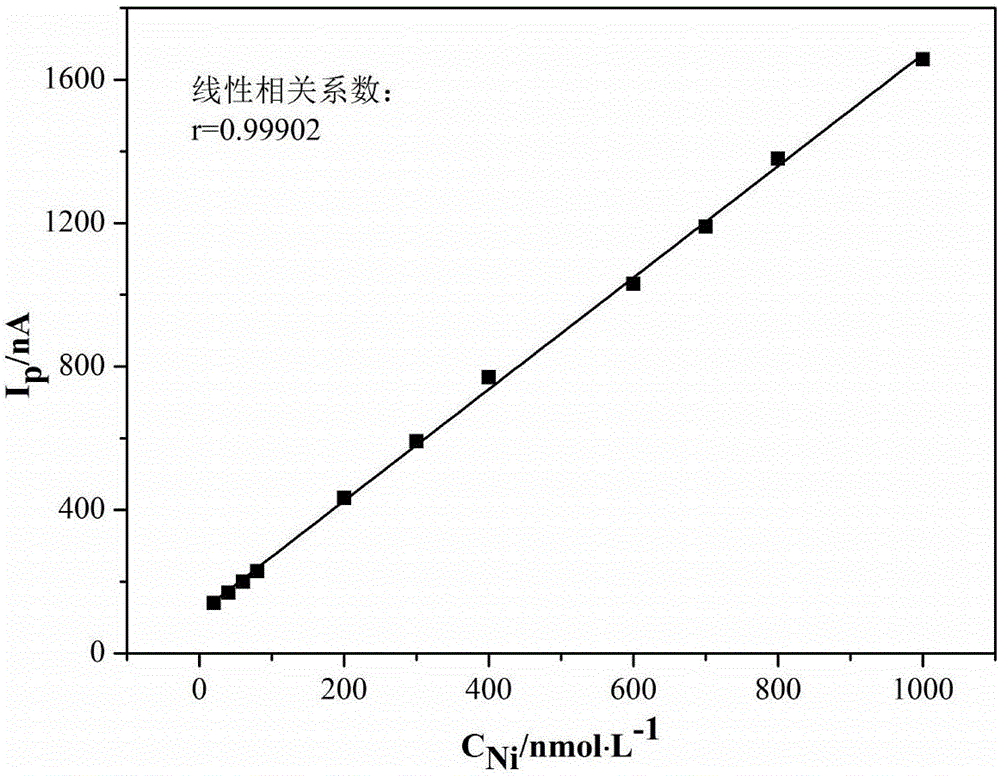
[0043] Example 1Cu 2+ 、Cd 2+ 、Ni 2+ and Co 2+ Determination of the standard curve of the assay system
[0044] (1) Take Cu 2+ The standard solution was diluted with double distilled water to the appropriate concentration of Cu 2+ Standard solution; measure 1×10 -6 mol / L, 2×10 -6 mol / L, 4×10 -6 mol / L, 6×10 -6 mol / L, 8×10 -6 mol / L, 2×10 -5 mol / L, 4×10 -5 mol / L, 8×10 -5 mol / L, 1×10 -4 mol / L, 2×10 -4 mol / L, 3×10 -4 mol / L, 6×10 -4 mol / L, 8×10 -4 mol / L Cu 2+ Put 0.5mL of each standard solution in 13 10mL volumetric flasks, add 1.0mL Zn to the volumetric flasks respectively 2+ solution (2.6mol / L) and 0.15mL dimethylglyoxime ethanol solution (11g / L), shake for 3min, then add 3mL sodium citrate solution (1mol / L), 4mL sodium borate (0.15mol / L) and 1mL Sodium hydroxide solution (2mol / L), constant volume, let stand for 5min, respectively pour the solution into the electrolytic cup to measure the produced Cu 2+ The complex absorbs the polarographic wave and obtains the ...
Embodiment 2
[0048] Cu in the new solution of embodiment 2 zinc electrolysis 2+ 、Cd 2+ 、Ni 2+ and Co 2+ Determination of content
[0049] (1) Measure 1mL of the new zinc electrolyte solution and place it in a 10mL volumetric flask, add 0.15mL of ethanol solution of dimethylglyoxime, shake for 3min until uniform, then add 3mL of sodium citrate solution (1mol / L) and 4mL of boric acid in sequence Sodium (0.15mol / L), adjust its pH value to 9.4 by adding 2mol / L sodium hydroxide solution dropwise, constant volume, let stand for 5min, pour the solution into the electrolytic cup to measure the Cu produced 2+ 、Ni 2+ 、Co 2+ The adsorption polarographic wave of the complex obtained its corresponding second-order derivative peak currents were 125.2nA, 667.1nA, and 214.7nA, and the detection results were as follows Figure 5 shown, and on this basis, 6mol / L sulfuric acid was added dropwise to adjust its pH value to 4.2, and the Cd produced was measured 2+ The adsorption polarographic wave of th...
Embodiment 3
[0052] Cu in the neutral supernatant of embodiment 3 2+ 、Cd 2+ 、Ni 2+ and Co 2+ Determination of content
[0053] (1) Measure 1 mL of neutral supernatant in a 100 mL volumetric flask, and distill it to volume with double distilled water, and use this as the solution to be tested;
[0054] (2) Measure 1mL of the solution to be tested in a 10mL volumetric flask, add 0.15mL of ethanol solution of dimethylglyoxime, shake for 3min until uniform, then add 3mL of sodium citrate solution (1mol / L) and 4mL of sodium borate (0.15mol / L), adjust its pH value to 9.3 by dropping 2mol / L sodium hydroxide solution, constant volume, let stand for 5min, pour the solution into the electrolytic cup to measure the Cu produced 2+ 、Ni2+ 、Co 2+ The adsorption polarographic wave of the complex obtained its corresponding second-order derivative peak currents of 22.5nA, 53nA, and 233.1nA, respectively, and the detection results are as follows Figure 9 shown, and on this basis, 6mol / L sulfuric acid ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com