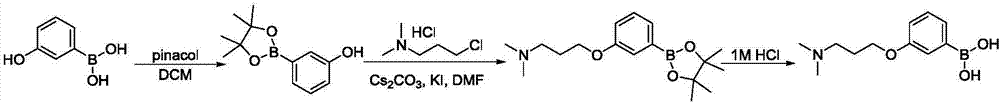
Preparation method of (3-(3-(dimethylamino) propoxy) phenyl) boronic acid
A technology of dimethylaminochloropropane and dimethylamino, which is applied in the field of preparation of propoxy)phenyl)boronic acid, can solve problems such as unsuitable for scale-up production, harsh reaction conditions, and high risk of reagents, and meets the reaction conditions and is easy Control, ease of purification, and ease of operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] A method for preparing (3-(3-(dimethylamino)propoxy)phenyl)boronic acid includes the following steps:
[0026] (1) Preparation of 3-hydroxyphenylboronic acid pinacol ester
[0027] Add 3-hydroxyphenylboronic acid (5g, 36.26mmol, 1.0eq) and dichloromethane (150mL) to a 500mL single-neck flask, add pinacol (5.1g, 43.51mmol, 1.2eq) under stirring, and stir at room temperature for 72h. The reaction solution was poured into water (200mL), the mixed solution was poured into a separatory funnel, the organic phase was separated, the aqueous phase was extracted twice with dichloromethane (200mL), the combined organic phases were dried with anhydrous sodium sulfate, filtered and evaporated to dryness The crude product was purified by column chromatography (200-300 mesh silica gel, dichloromethane / methanol mixed solvent with a volume ratio of 100 / 1 as the eluent) to obtain 7.0 g of white solid with a yield of 88% and a purity of 96%.
[0028] (2) Preparation of (3-(3-(dimethylamino)prop...
Embodiment 2
[0034] A method for preparing (3-(3-(dimethylamino)propoxy)phenyl)boronic acid includes the following steps:
[0035] (1) Preparation of 3-hydroxyphenylboronic acid pinacol ester
[0036] Add 3-hydroxyphenylboronic acid (10.0g, 72.46mmol, 1.0eq) and dichloromethane (300mL) to a 500mL single-neck flask, add pinacol (10.0g, 84.60mmol, 1.2eq) under stirring, and stir at room temperature for 48h The reaction solution was poured into water (400mL), the mixed solution was poured into a separatory funnel, the organic phase was separated, the aqueous phase was extracted twice with dichloromethane (400mL), the combined organic phase was dried with anhydrous sodium sulfate, filtered and evaporated Dry, the crude product was purified by column chromatography (200-300 mesh silica gel, dichloromethane / methanol mixed solvent with a volume ratio of 100 / 1 as eluent) to obtain 15.0 g of white solid with a yield of 94% and a purity of 97%.
[0037] (2) Preparation of (3-(3-(dimethylamino)propoxy)phen...
Embodiment 3
[0042] A method for preparing (3-(3-(dimethylamino)propoxy)phenyl)boronic acid includes the following steps:
[0043] (1) Preparation of 3-hydroxyphenylboronic acid pinacol ester
[0044] Add 3-hydroxyphenylboronic acid (50.0g, 362.32mmol, 1.0eq) and dichloromethane (1500mL) to a 3000mL three-necked flask, add pinacol (51.0g, 431.47mmol, 1.2eq) under stirring, and stir at room temperature for 96h The reaction solution was poured into water (2000mL), the mixed solution was poured into a separatory funnel, the organic phase was separated, the aqueous phase was extracted twice with dichloromethane (2000mL), the combined organic phase was dried with anhydrous sodium sulfate, filtered and evaporated Dry, the crude product was purified by column chromatography (200-300 mesh silica gel, dichloromethane / methanol mixed solvent with a volume ratio of 100 / 1 as eluent) to obtain 75.0 g of white solid, with a yield of 94% and a purity of 96%.
[0045] (2) Preparation of (3-(3-(dimethylamino)prop...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com