Electrically conductive material and laminate
A raw material and polymer conductive technology, applied in the direction of conductive coatings, electronic equipment, non-metallic conductors, etc., can solve the problem that the layer adhesion strength of conductive durability filmization cannot be said to be sufficient, and achieve excellent conductive durability, Excellent conductive durability, excellent strength effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
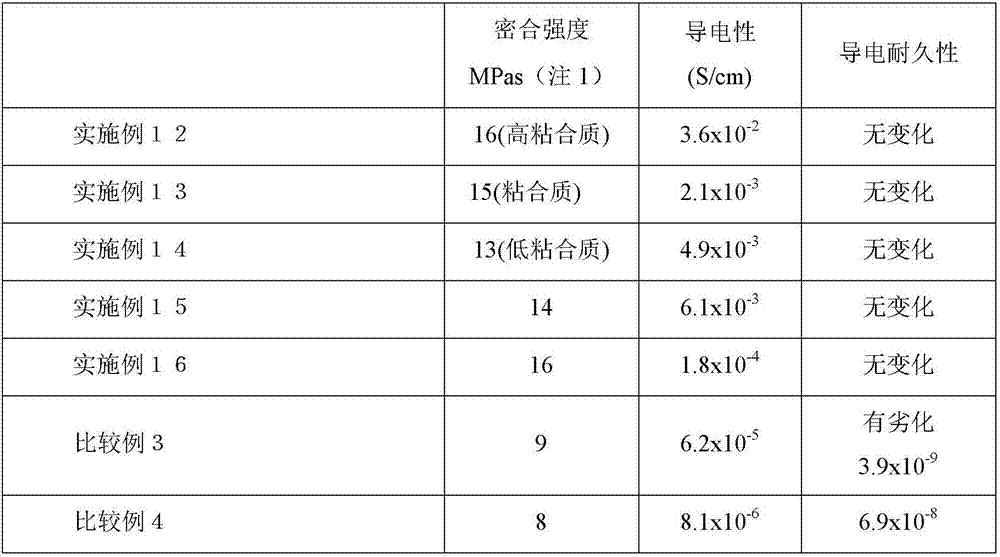
Embodiment 1
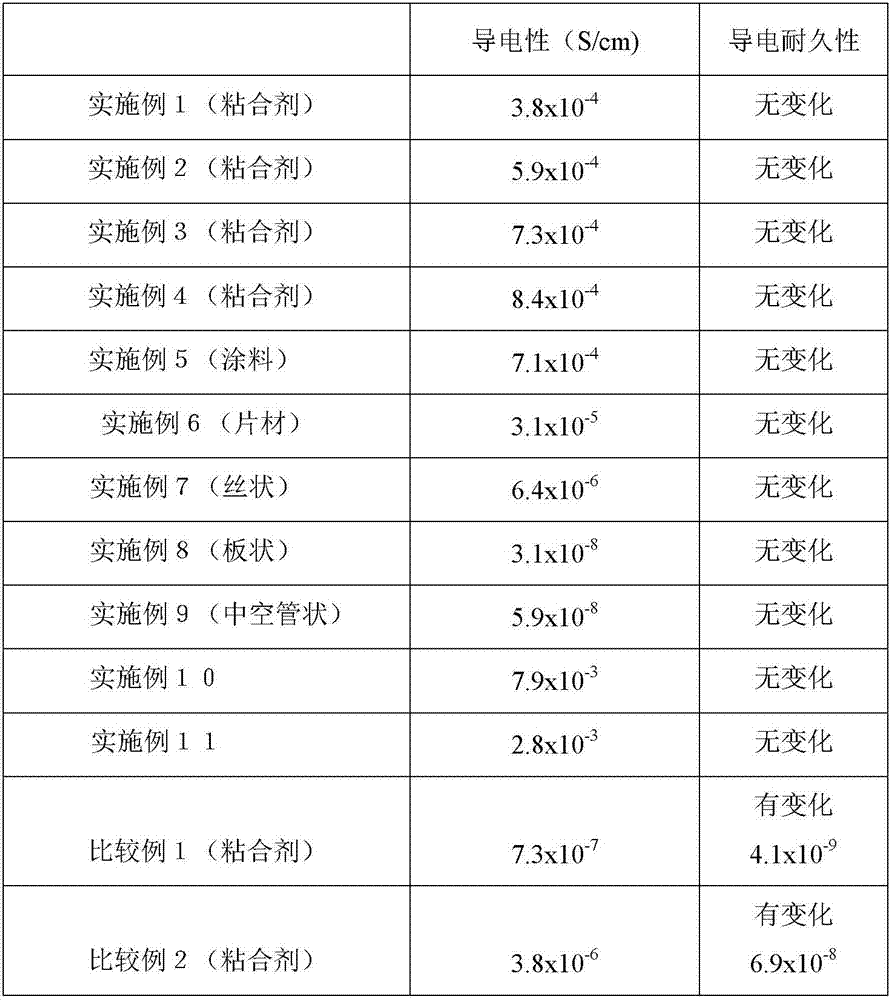
[0129] Embodiment 1 (conductive raw material=adhesive)
[0130] In the above-mentioned graft polymer 1 {polymer conductive composition (X 1 )}30% by weight, fluoropolymer (polyvinylidene fluoride) (X 2 )70% by weight, will be relative to (X 1 ) and (X 2 ) with a total of 10% by weight of a dispersant phthalocyanine (a hydroxyl-containing petroleum resin "Lionol Blue" produced by Toyo Ink Manufacturing Co., Ltd.) was melt-mixed into N-methylpyrrolidone to obtain a conductive bond with a solid content of 10% by weight agent.
Embodiment 2
[0131] Embodiment 2 (conductive raw material=adhesive)
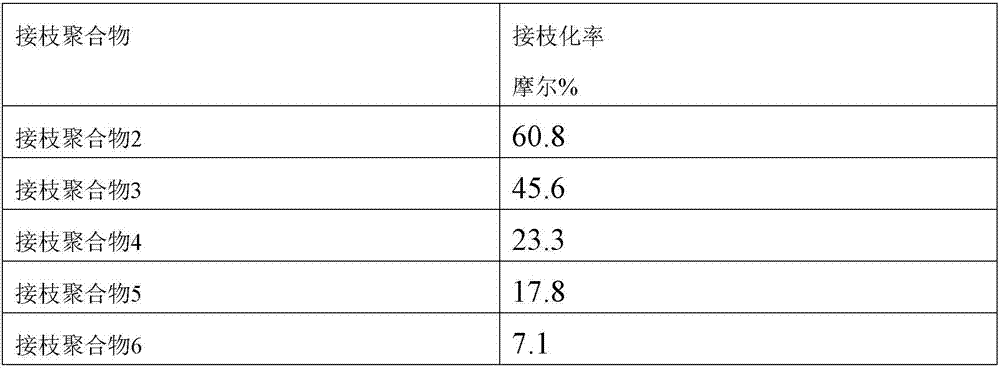
[0132] In the above-mentioned graft polymer 2 (polymer electrolyte composition) (X 1 )10% by weight, fluoropolymer (polyvinylidene fluoride) (X 2 ) 20% by weight, acrylic resin (trade name "BR-106" manufactured by Mitsubishi Rayon Co., Ltd.) 70% by weight (Y), will be relative to (X 1 ) and (X 2 ) with a total of 10% by weight of a dispersant phthalocyanine (a petroleum resin containing a hydroxyl group "LionolBlue" produced by Toyo Ink Manufacturing Co., Ltd.) was melt-mixed in N-methylpyrrolidone to obtain a conductive adhesive with a solid content of 10% by weight .
Embodiment 3
[0133] Embodiment 3 (conductive original material=adhesive)
[0134] In the above-mentioned graft polymer 3 (polymer electrolyte composition) (X 1 )20% by weight, fluoropolymer (polyvinylidene fluoride) (X 2 ) 70% by weight, molten salt {1-ethyl-3-methylimidazolium tetrafluorosulfonimide (EMI-TFSI)} 10% by weight without a polymerizable group, relative to (X 1 ) Adding 1.0 molar charge transfer ion source (lithium tetrafluorosulfonimide) will be relative to (X 1 ) and (X 2 ) and 10% by weight of dispersant phthalocyanine (trade name: Hydroxyl-containing petroleum resin "Lionol Blue" produced by Toyo Ink Manufacturing Co., Ltd.) in total of 10% by weight of molten salts without polymerizable groups were melt-mixed in N-methylpyrrolidone , to obtain a conductive adhesive with a solid content of 10% by weight.
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com