Organic second-order nonlinear optical chromophore having double-donor structure, and synthesis method and application thereof
A second-order nonlinear and chromophore technology, which is applied in the field of organic second-order nonlinear optical chromophore and its synthesis, can solve the problem of low polarization efficiency, failure to meet device requirements, and low electro-optic coefficient And other issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
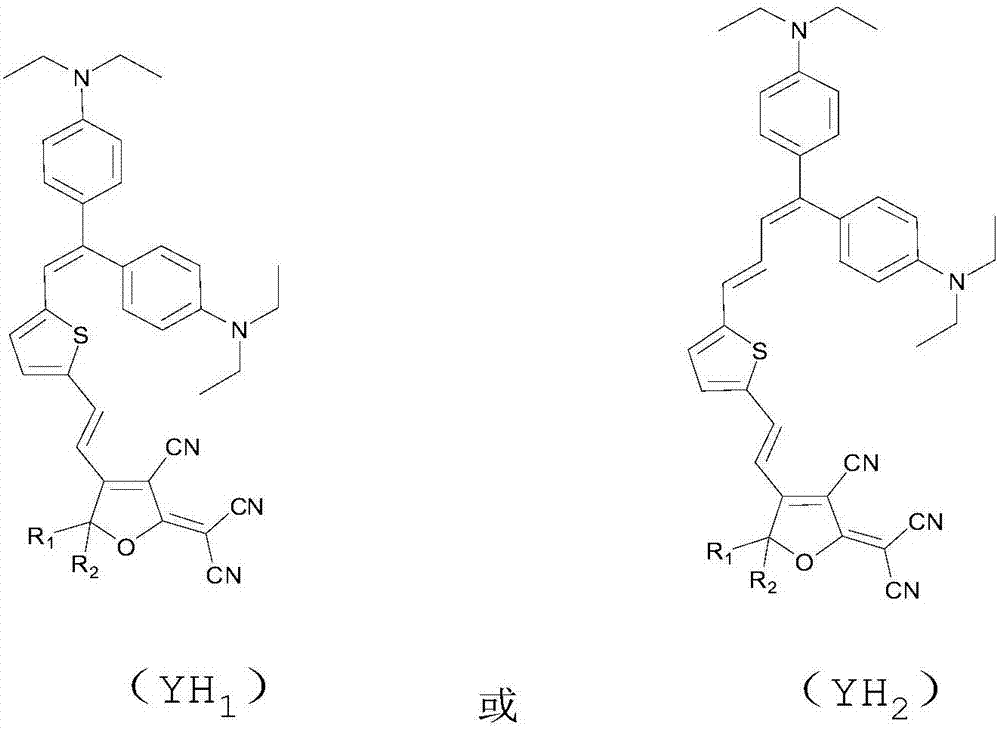
[0055] An organic second-order nonlinear optical chromophore with a dual-donor structure was synthesized as shown below:
[0056]
[0057] The synthetic route is as follows (formula 1):
[0058]
[0059] The synthesis method is:
[0060] S1: Synthesis of compound 2 shown in (Formula 1)
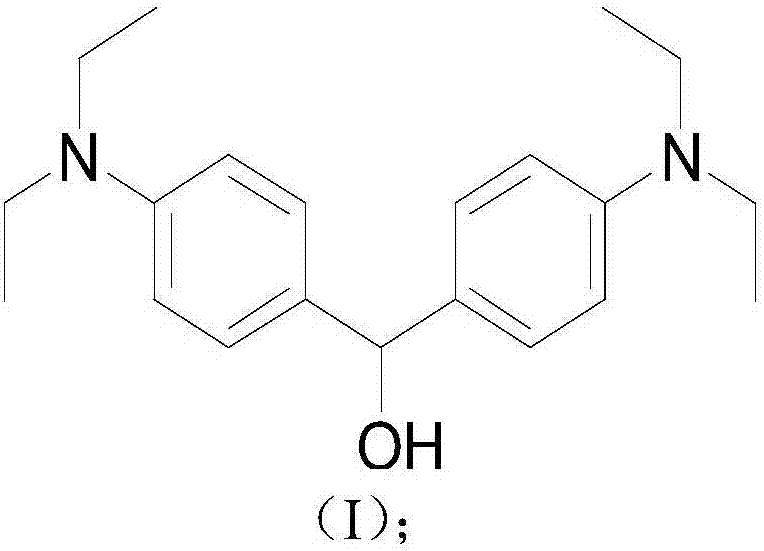
[0061] 3.25g (10mmol) tetraethyl Michler's ketone was added to redistilled THF, and then 0.95g (25mmol) NaBH 4 Add in batches, react at room temperature for 12h, after the reaction is completed, pour the reaction product into 100ml deionized water; then extract with ethyl acetate, and use anhydrous MgSO 4 The organic phase was dried, and ethyl acetate was removed by rotary evaporation to obtain 2.85 g of compound 2. The compound 2 is a white solid. This alcohol is unstable, and the next reaction should be carried out as soon as possible.
[0062] S2: Synthesis of compound 3 shown in (Formula 1):
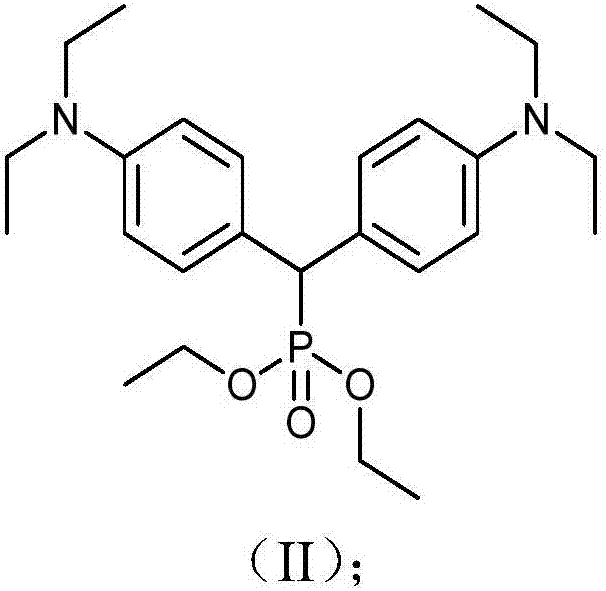
[0063] 2.78g (8.53mmol) of compound 2 and 9ml (54mmol) of triethyl phosphate were fir...
Embodiment 2
[0079] An organic second-order nonlinear optical chromophore with a dual-donor structure was synthesized as shown below:
[0080]
[0081] The synthetic route is as follows:
[0082]
[0083] The synthesis method is:
[0084] S11: Synthesis of Compound 2 shown in (Formula 2)
[0085] N 2 Under protection, 0.48g (0.02mol) of sodium hydride (NaH) was dissolved in an appropriate amount of redistilled THF, cooled to 0 degrees in an ice-salt bath, and 3.26g (0.02mol) of cyanomethylphosphoric acid was added dropwise at this temperature ester, when the solution becomes clear, add 3.24g (0.01mol) of tetraethyl Michler's ketone, then heat to reflux for 12h, pour the reaction result into 100ml deionized water; then extract with ethyl acetate, and use anhydrous MgSO 4 The organic phase was dried, ethyl acetate was removed by rotary evaporation, and 2.25 g of compound 2 was obtained by column chromatography. The compound 2 is a yellow solid.
[0086] MS (MALDI-TOF), m / z: 347.2...
Embodiment 3
[0101] synthetic polymer film
[0102] (1) Add 0.075g of amorphous polycarbonate (APC) into 1.00mL of dibromomethane, stir for 3-5h until APC is completely dissolved, then add 0.025g of the organic second-order nonlinear optical chromophore synthesized in Example 1 (YH 3 ), to obtain the mixed solution of organic second-order nonlinear optical chromophore compound and APC, the mixed solution obtained is coated with spin coating method on the ITO glass substrate (controlling speed is 800~1200 revs / min), then at 60 °C in a vacuum oven for 24 hours to obtain a polymer film I. The thickness of the polarized polymer film is 2-4 μm.
[0103] (2) Add 0.075g of amorphous polycarbonate (APC) into 1.00mL of dibromomethane, stir for 3-5h until APC is completely dissolved, then add 0.025g of the organic second-order nonlinear optical chromophore synthesized in Example 2 (YH 4 ) to obtain a mixed solution of organic second-order nonlinear optical chromophore compound and APC, which is ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com