Application of lettuce as host in expressing protein and/or polypeptide
A protein and lettuce technology, applied in animal/human peptides, biochemical equipment and methods, peptides, etc., can solve problems such as difficult purification processes
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
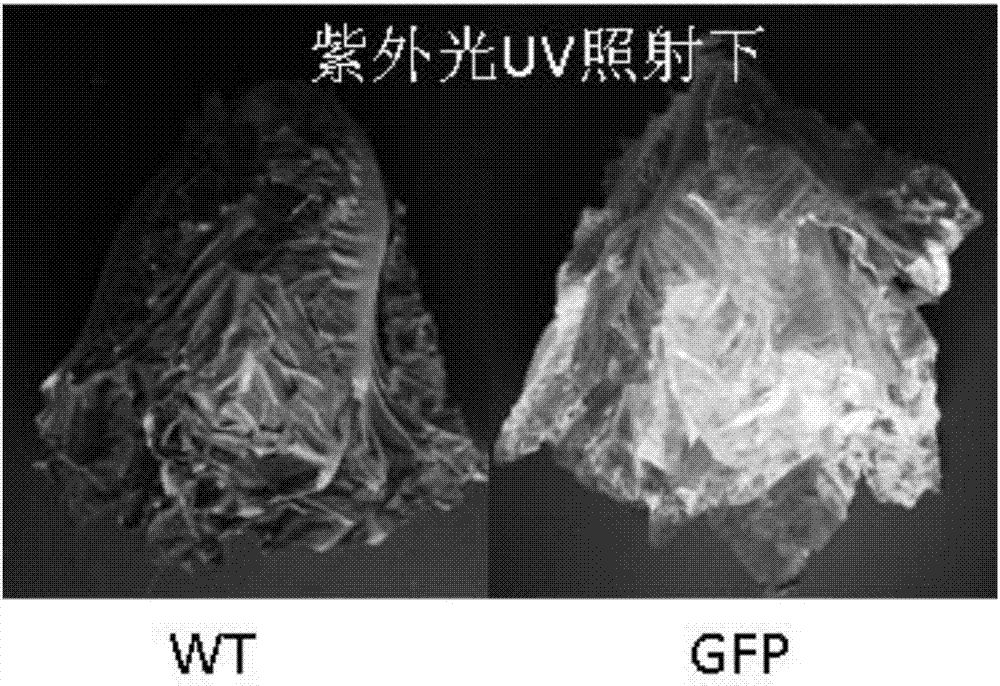
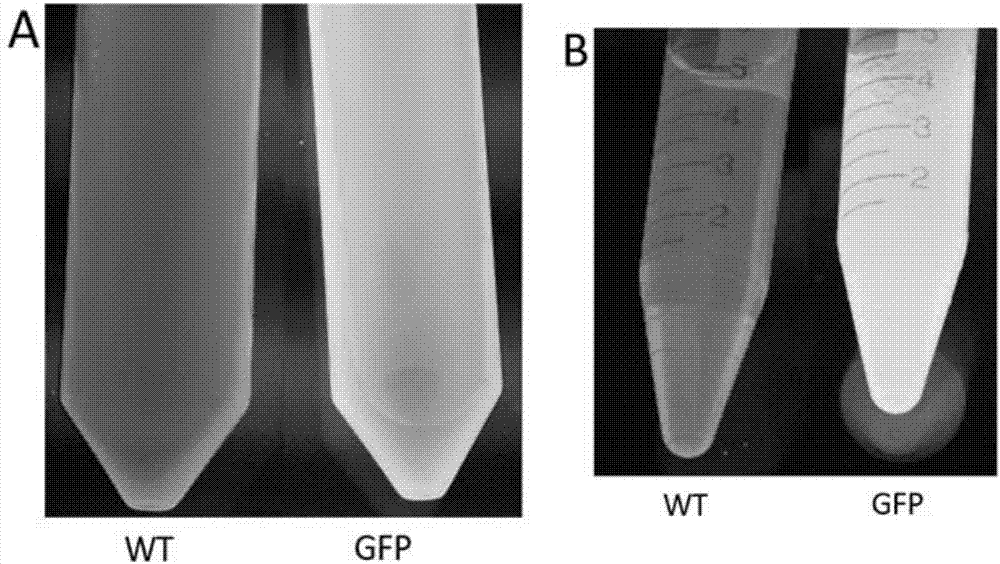
[0048] Embodiment 1 utilizes lettuce system to produce green fluorescent protein (GFP)
[0049] Tissue and Agrobacterium tumefaciens preparation:
[0050] Mature lettuces (red leaves, green leaves and long leaves) were purchased from a local vegetable store and cultivated under a standard growing lamp for 12 hours; or the lettuce seeds were cultivated without soil as shown in Figure 2(B). The binary plant vector pCambia1302 (containing GFP, figure 1 ) Agrobacterium tumefaciens strain LBA 4404 was inoculated into 0.5L YEB (yeast extract broth, 5g / L sucrose, 5g / L tryptone, 6g / L yeast extract, 0.24g / L MgSO 4 , pH7.2) and supplement antibiotic liquid medium (50mg / L kanamycin). The inoculated culture was incubated in a shaker (220 rpm) at 25-28°C for 72h. The OD600 value was measured and adjusted to 3.5-4.5 by adding YEB medium. Then the culture solution was collected and centrifuged (4500 rpm) for 10 min. Resuspend Agrobacterium cells in osmotic medium (10mM MES, 10mM MgSO 4...
Embodiment 2
[0061] Embodiment 2 utilizes lettuce system to produce lumbrokinase
[0062] Plant expression constructs:
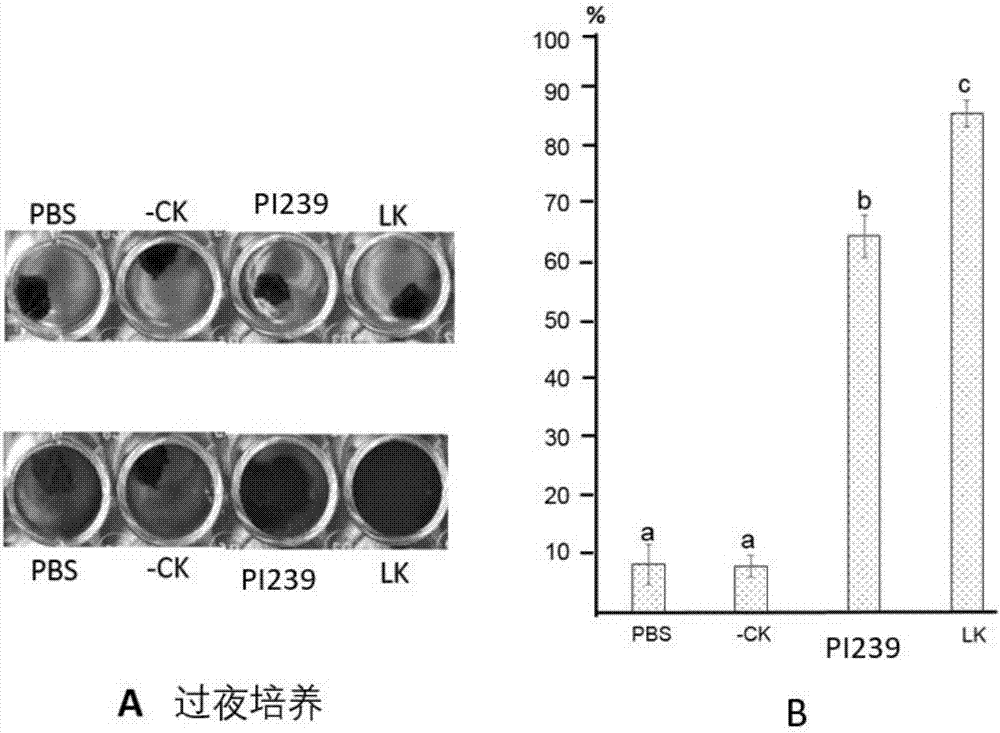
[0063] Based on the full-length PI239DNA sequence of GenBank (Accession no: AF433650), we ligated the restriction site XbaI site to the 5' end of PI239, and added a BamH1 site to the 3' end of the fragment, provided by GenscriptInc (Piscataway, NJ, USA) and cloned into the pUC57 vector to obtain the cloning vector pUC-Lum. The fragment PI239 was isolated from pUC-Lum by KpnI / SacI, and cloned into the plant binary vector pCam35S (preserved in our laboratory) to generate the plant expression vector p35-Lum. The plant expression vector p35-Lum was subsequently electroporated into Agrobacterium tumefaciens BA4404 by Multiporator (Eppendorf, Hamburg, Germany) for subsequent transient expression assays in lettuce. The size of the purified recombinant lumbrokinase protein was observed by SDS-PAGE gel electrophoresis.
[0064] Fibrinolytic Activity Assay:
[0065]Agarose (0....
Embodiment 3
[0071] Control group: using tobacco leaves to produce green fluorescent protein and lumbrokinase;
[0072] Experimental group: lettuce provided by the invention produces green fluorescent protein and lumbrokinase;
[0073] Table 1 Green fluorescent protein and lumbrokinase
[0074]
[0075] * Compared with the control group, P≤0.05; # Compared with the control group, P≤0.01;
[0076] It can be seen from Table 1 that compared with the tobacco leaf system of the control group, lettuce transiently expresses green fluorescent protein and lumbrokinase, which significantly (P≤0.05) shortens the production cycle, significantly (P≤0.05) increases the protein content, and significantly (P≤0.05) increases the protein content. 0.05) improves the protein activity, simplifies the difficulty of protein purification, and significantly (P≤0.01) reduces the production cost.
[0077] Based on the above test results, the plant system, especially the lettuce system is a more economical and...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com