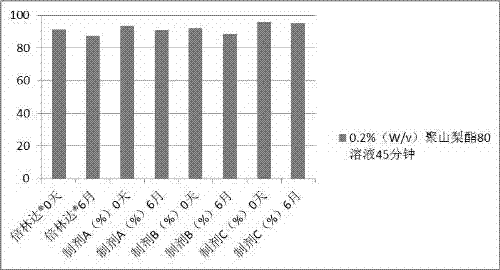
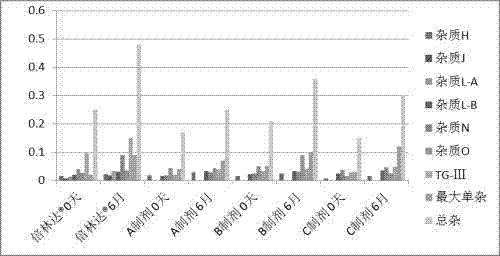
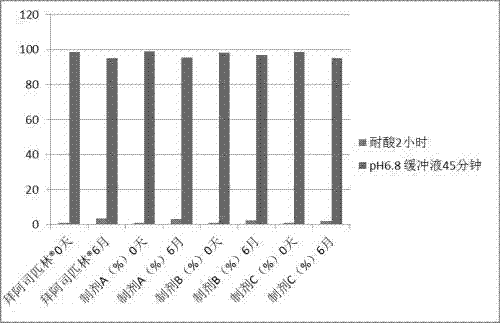
Preparation method of compound oral solid preparation containing Ticagrelor
A technology for ticagrelor and solid preparations, which is applied in the field of preparation of ticagrelor compound oral solid preparations, can solve problems such as increased incidence of gastrointestinal side effects, and achieves improved compliance, scientific preparation method, and compound specification setting. reasonable effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0064] Example 1 (Tablet - Formulation A)
[0065]
[0066] Preparation:
[0067] a. Preparation of aspirin enteric-coated tablets
[0068]Mix aspirin with microcrystalline cellulose, pregelatinized starch, and stearic acid evenly and then directly compress into tablets; prepare the isolation coating layer solution with Opadry film coating powder; mix Eudragit L30D-55 (30% water dispersion Glyceryl monostearate and triethyl citrate were added to the body) and stirred evenly to prepare an enteric-coated layer solution. Use a high-efficiency coating pan to coat the plain tablets with an isolation coating and an enteric coating, and dry them in an oven at 40°C for 24 hours.
[0069] b. Preparation of ticagrelor granules:
[0070] Crush ticagrelor to particle size D 90 <85μm, then mixed with mannitol, calcium hydrogen phosphate, carboxymethyl starch sodium, wet granulation with hydroxypropyl cellulose solution as binder, dried, and then mixed with external magnesium stearat...
Embodiment 2
[0073] Example 2 (Capsules - Formulation B)
[0074]
[0075] Preparation:
[0076] a. Preparation of aspirin enteric-coated pellets
[0077] Mix aspirin and microcrystalline cellulose, use hypromellose and povidone as binders, and use an extrusion spheronization process to prepare active drug pellet cores. Then prepare Opadry film coating powder into film coating solution; dissolve Eudragit L100-55 in 95% ethanol, add triethyl citrate and stir evenly, then pour the suspension of talcum powder into In the L100-55 solution, the enteric-coated layer solution was prepared, and then the film coating layer and the enteric-coated layer were sprayed on the upper drug pellets by fluidized bed spraying technology to make aspirin enteric-coated pellets, and dried in an oven at 40°C Available within 24 hours.
[0078] b. Preparation of ticagrelor pellets:
[0079] Crush ticagrelor to particle size D 90 <85μm, and then mixed with microcrystalline cellulose and sodium carboxymethyl...
Embodiment 3
[0084] Example 3 (Tablet - Formulation C)
[0085]
[0086] Preparation:
[0087] a. Preparation of blank pellet core
[0088] The microcrystalline cellulose blank pellet core was used as the blank pellet core.
[0089] b. Preparation of aspirin enteric-coated pellets
[0090] The aspirin is dissolved in the ethanol solution of povidone to prepare the drug-containing layer solution. Mix Eudragit L30D-55 (30% water dispersion) and Eudragit NE30D (30% water dispersion) in proportion, then add glycerol monostearate and triethyl citrate and stir evenly to prepare Enteric layer solution. Opadry film coating powder is used to prepare film isolation coating solution. The drug-containing layer solution is sprayed onto the core of blank pellets by fluidized bed spraying technology to prepare drug-coated pellets. Then spray the coating solution of the isolation layer on the upper drug pellets, and then spray the enteric coating layer to make aspirin enteric-coated pellets, and ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com