Deoxynivalenol aptamer affinity column as well as preparation method and application thereof
A technology of aptamers and affinity columns, applied in chemical instruments and methods, separation methods, preparation of test samples, etc., can solve problems such as poor linearity of standard curves, large coefficient of variation, matrix interference, etc., to reduce Cross-reactivity, high purification efficiency, and high-affinity effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] Example 1: Preparation of affinity column using amino-modified deoxynivalenol aptamer
Embodiment approach
[0041] A preferred embodiment of the present invention to prepare deoxynivalenol aptamer affinity column is as follows:
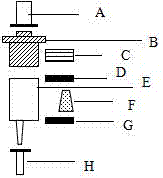
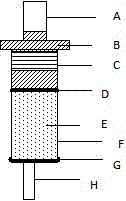
[0042] 1. Vector activation
[0043] Select N-hydroxysuccinimide (NHS) modified agarose carrier sepharose4B for activation
[0044] Take 1g of N-hydroxysuccinimide-modified agarose, add 50ml of 1mM HCl, after swelling for 30min, wash the gel with 100ml of 1mM HCl for 6 times, and then wash with 100ml of ultrapure water
[0045] 2. The activated agarose gel sepharose4B with coupling buffer (0.1M NaHCO 3 , 0.8M NaCl, pH8.2) and washed 3 times. Add 20mol / L amino-modified deoxynivalenol aptamer, and couple at room temperature for 8 hours
[0046] 3. Wash the coupled deoxynivalenol aptamer-agarose carrier with 20mM, pH7.4 phosphate buffer PBS for 3 times
[0047] 4. closed
[0048] Add 100mmol / L Tris.ClPH8.0 to the coupled deoxynivalenol aptamer-agarose carrier, and react at room temperature for 2 hours
[0049] 5. Wash the blocked deoxynivalenol aptamer-a...
Embodiment 2
[0060] Example 2: Preparation of an affinity column using biotin-modified deoxynivalenol aptamer
[0061] A preferred embodiment of the present invention to prepare deoxynivalenol aptamer affinity column is as follows:
[0062] 1. Take 1ml streptavidin (SA)-modified agarose gel sepharose4B, wash 3 times with 10ml ultrapure water
[0063] 2. Wash the (SA) modified sepharose4B three times with coupling buffer (0.02M PBS, 0.8M NaCl, pH7.4). Add 20mol / L of biotin-modified deoxynivalenol aptamer, and couple at room temperature for 4 hours
[0064] 3. Wash the coupled deoxynivalenol aptamer-agarose carrier with 20mM, pH7.4 phosphate buffer PBS for 3 times
[0065] 4. Column packing
[0066] Put the deoxynivalenol-agarose carrier into the chromatographic column according to the needs, and the deoxynivalenol affinity purification columns of different capacities can be prepared according to the needs
[0067] 1) Take the empty cylinder, add the lower sieve plate, add 1ml PBS, let i...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com