Carbon-nitrogen catalyst for photosynthesizing hydrogen peroxide and preparation method thereof
A technology for photosynthesis of hydrogen peroxide and carbon nitrogen catalysts, which is applied in the direction of catalyst activation/preparation, physical/chemical process catalysts, chemical instruments and methods, etc. It can solve the problems of environmental pollution, high toxicity, and high energy consumption in the production process. Achieve the effect of broadening the photoresponse range and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
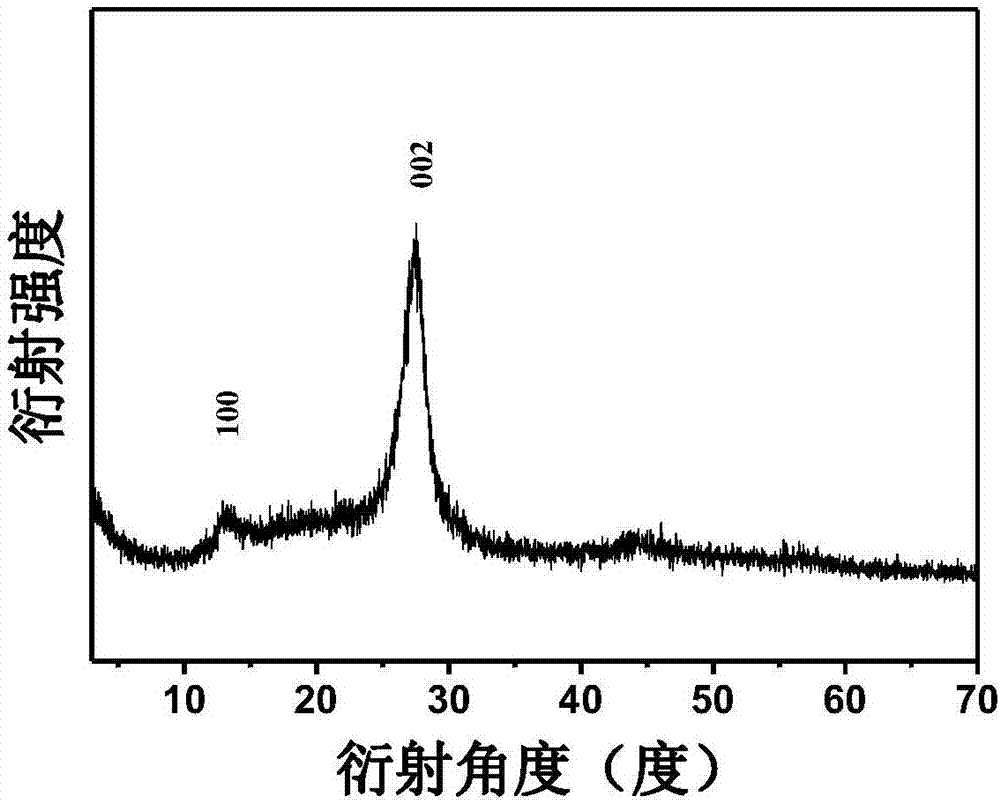
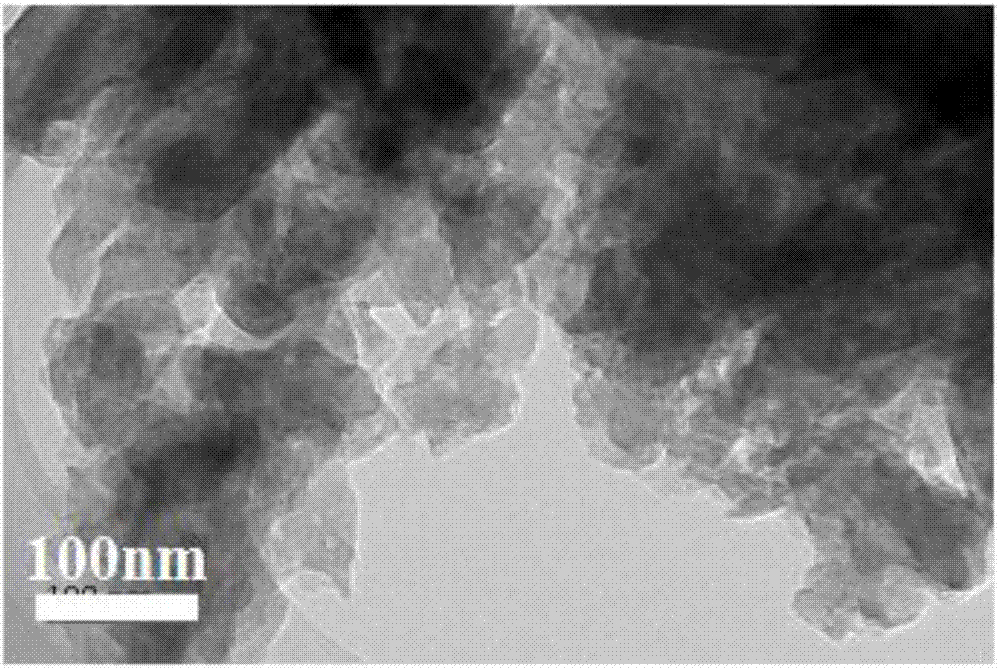
[0028] A. Weigh 3 g of melamine and grind it in a mortar for 10 minutes, then put it into a muffle furnace and roast it in a nitrogen atmosphere, raise the temperature to 520°C at a heating rate of 5°C / min, keep it warm for 2 hours, and then cool it down at a rate of 5°C / min Rate is cooled to room temperature, obtains pale yellow solid powder, and it is placed in the mortar and grinds 30min, obtains C 3 N 4 .
[0029] B. The C obtained in step A 3 N 4 Put it in a glucose solution with a concentration of 0.03mol / L, ultrasonic for 4 hours; put it in a hydrothermal reactor, react at 120°C for 12 hours, cool down to room temperature, take it out and centrifuge at 5000 rpm for 5 minutes, and then deionize it with 100mL Water washing, centrifugation and deionized water washing steps were repeated 3 times, and then dried in an oven at 70°C for 12 hours.
[0030] C. Roast the obtained sample in a nitrogen atmosphere, raise the temperature to 500°C at a heating rate of 5°C / min, kee...
Embodiment 2
[0032] A. Weigh 10g of melamine and grind it in a mortar for 20min, then put it into a muffle furnace and roast it in a nitrogen atmosphere, raise the temperature to 500°C at a heating rate of 4.5°C / min, keep it warm for 2.5 hours, and then cool it down at a rate of 8°C / min Rate is cooled to room temperature, obtains pale yellow solid powder, and it is placed in the mortar and grinds 60min, obtains C 3 N 4 .
[0033] B. The C obtained in step A 3 N 4 Put it in a glucose solution with a concentration of 0.01mol / L, ultrasonicate for 4.5 hours; put it in a hydrothermal reaction kettle, react at 125°C for 11 hours, cool down to room temperature, take it out and centrifuge it at 6000 rpm for 8 minutes, and then use 120mL deionized The water washing, centrifugation and deionized water washing steps were repeated 3 times, and then dried in an oven at 70°C for 10 hours.
[0034] C. Roast the obtained sample in a nitrogen atmosphere, raise the temperature to 500°C at a heating rate...
Embodiment 3
[0037] A. Weigh 15g of melamine and grind it in a mortar for 15min, then put it into a muffle furnace and bake it in a nitrogen atmosphere, raise the temperature to 550°C at a heating rate of 5°C / min, keep it warm for 2.5 hours, and then cool it down at a rate of 10°C / min Rate is cooled to room temperature, obtains light yellow solid powder, it is placed on the mortar and grinds 50min, obtains C 3 N 4 .
[0038] B. The C obtained in step A 3 N 4 Put it in a glucose solution with a concentration of 0.006mol / L, ultrasonicate for 5 hours; put it in a hydrothermal reactor, react at 120°C for 10 hours, cool down to room temperature, take it out and centrifuge it at 5500 rpm for 7 minutes, and then use 110mL deionized The water washing, centrifugation and deionized water washing steps were repeated 3 times, and then dried in an oven at 70°C for 9 hours.
[0039] C. Roast the obtained sample in a nitrogen atmosphere, raise the temperature to 520°C at a rate of 5°C / min, keep it wa...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com