Bifunctional glutathione synthetase mutant, and nucleotide sequence, preparation method and application thereof
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0090] The present invention also provides a method for preparing the above-mentioned bifunctional glutathione synthetase mutant, comprising the following steps:
[0091] One or more of random mutation, saturation mutation and iterative saturation is performed on the wild-type GshF nucleotide sequence of Streptococcus salivarius of SEQ ID NO: 1 to obtain the nucleotide sequence encoding the mutant GshF as before.
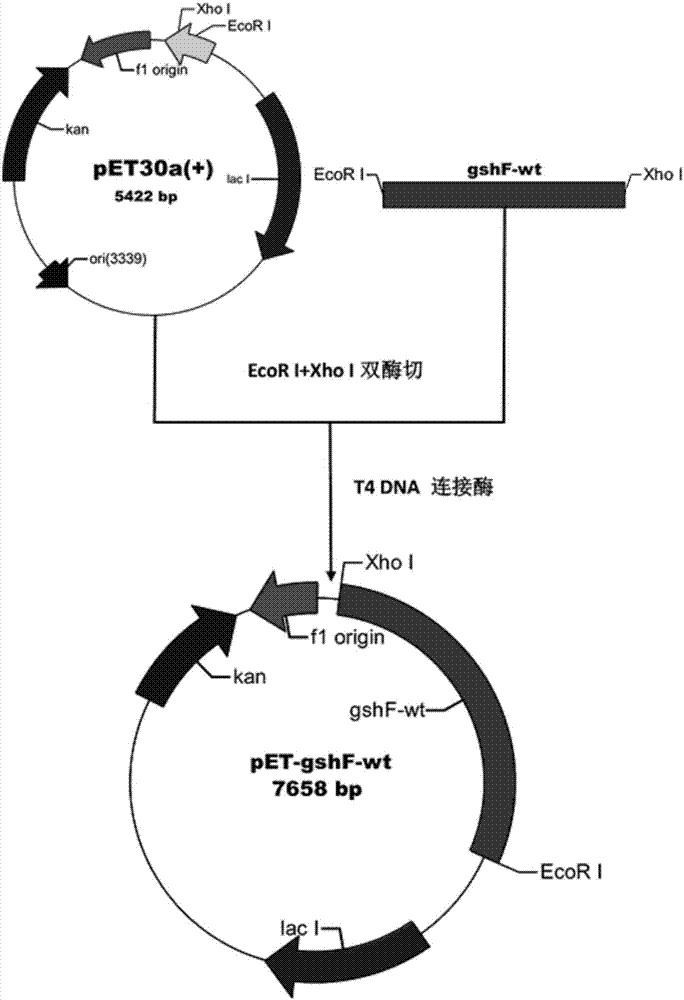
[0092] The nucleotide sequence of the mutant is inserted into the vector to obtain a recombinant expression vector.
[0093] The recombinant expression vector is transformed into the expression strain to obtain the recombinant genetically engineered bacteria.
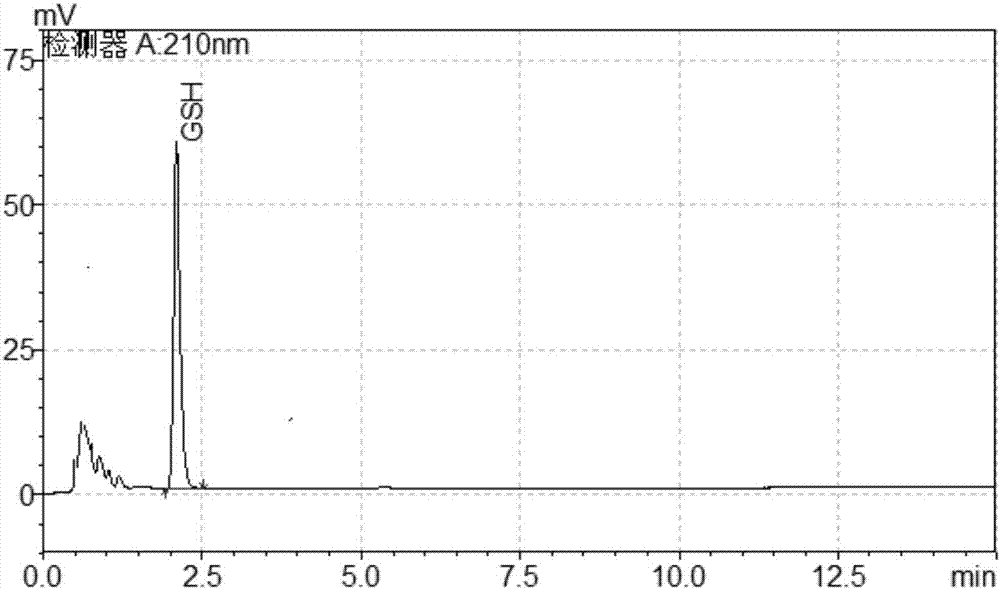
[0094] The recombinant genetically engineered bacteria are induced by fermentation, crushed, and isolated to obtain a bifunctional glutathione synthetase mutant.
[0095] The wild-type GshF nucleotide sequence of Streptococcus salivarius with SEQ ID NO: 1 in the sequence listing is subjected to steps such as...
Embodiment 1
[0102] Example 1: Construction and expression of wild-type GshF recombinant genetically engineered bacteria and purification and immobilization of recombinant proteins
[0103] 1-1 Obtaining of wild-type GshF gene
[0104] In order to obtain a mutant GshF with high synthetic activity, weak product inhibition, strong heat resistance and good operational stability, the wild-type GshF gene and amino acid sequence used in the present invention are derived from Streptococcus salivarius (GeneBank accession number: WP_038676473 ), the coding gene was optimized through Escherichia coli codon bias and the whole gene synthesis was carried out, and the wild-type bifunctional glutathione synthetase was named GshF-WT, and its coding gene was named gshF-wt. The following nucleotide sequence and amino acid sequence are shown in SEQ ID NO:1 and SEQ ID NO:2.
[0105] 1-2 Construction of wild-type GshF prokaryotic expression vector and construction of recombinant genetically engineered bacteri...
Embodiment 2
[0117] Embodiment 2: Preparation of GshF mutants
[0118] 2-1 Preparation of GshF mutant with high synthetic activity
[0119] Construction of 2-1-1GshF mutant library
[0120] In order to improve the synthetic activity of wild-type GshF, the inventors used the recombinant expression vector pET-gshF-wt as a DNA template, and the primers were T7 universal primers (SEQ ID NO: 15 and 16), and constructed a random GshF by error-prone PCR method. Mutant library, and by adjusting the concentration of Mg2+ and Mn2+ and the concentration of dCTP and dTTP oligonucleotides in the error-prone PCR reaction system, the base mismatch rate of the mutant library is five per thousand, that is, to ensure that a mutant has 1 to 3 amino acids are mutated, and the specific process of constructing the mutant library is as follows.
[0121] Error-prone PCR reaction system:
[0122] 10×Buffer
5μL
2mmol / L dNTPS
5μL
100mmol / L dCTP
0.5μL
100mmol / L dTTP
0....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com