Photosensitive dye with d-d-π-a structure using triphenylamine as a double electron donor and its preparation method and application
A technology of electron donor and photosensitive dye, which is applied in the field of solar cells to achieve the effect of low preparation cost, high photoelectric conversion efficiency and less process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
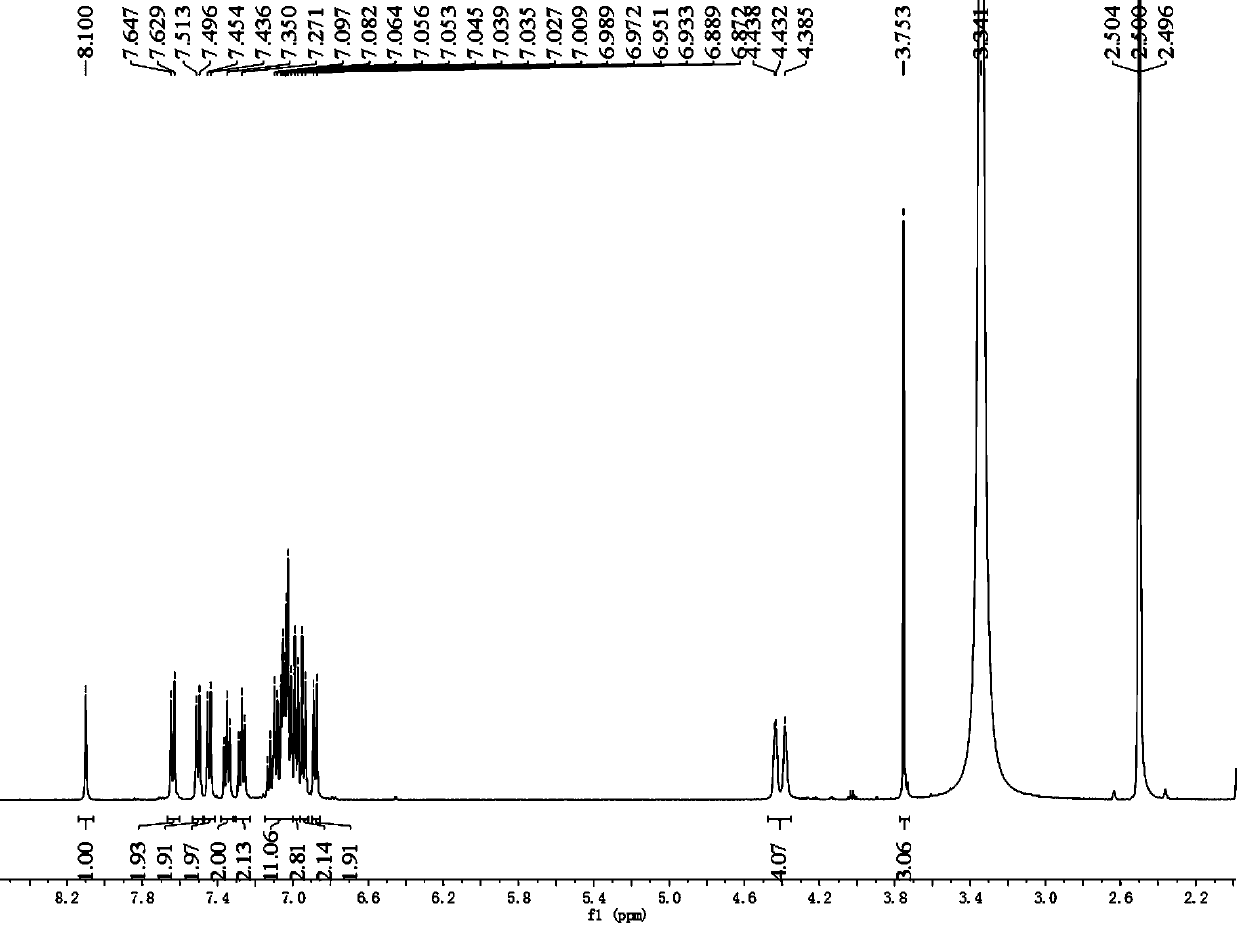
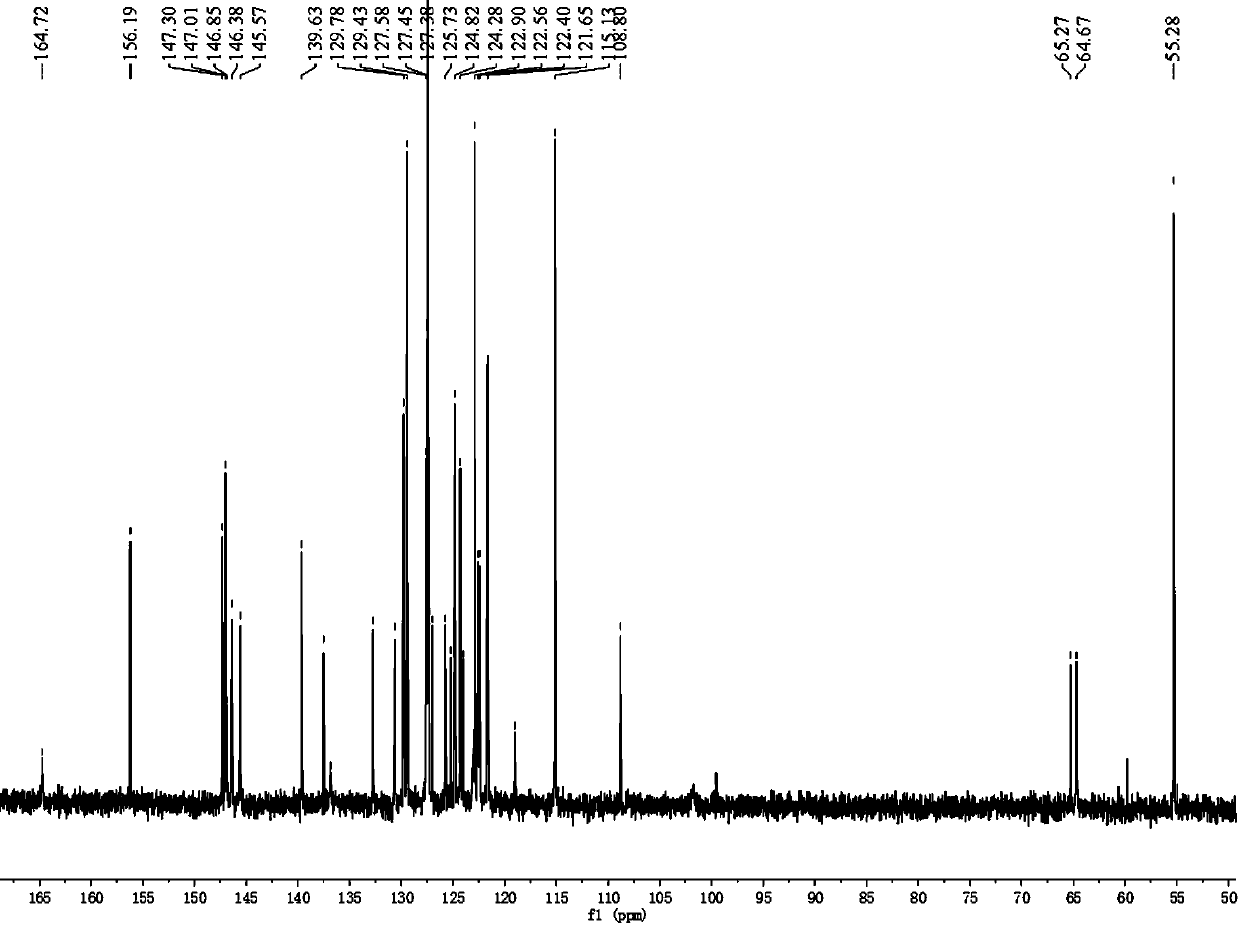
[0070] Example 1 Photosensitizing dye I (2-cyano-3-((2-(4-(2-(4-methoxytriphenylamine))vinyl)triphenylamine) 3,4-ethylenedioxythiophene) Acrylic acid) and preparation method thereof
[0071] Photosensitizing dye Ⅰ uses triphenylamine as a double electron donor, EDOT as a π-conjugated bridge, and cyanoacetic acid as an electron acceptor. Its chemical structural formula is: Its synthetic route is as follows:
[0072]
[0073] The preparation method of photosensitizing dye I comprises the following steps carried out in sequence:
[0074] (1) Synthesis of Intermediate 1
[0075] Add 6g of 3,4-ethylenedioxythiophene and 150mL of anhydrous tetrahydrofuran into a 500mL four-necked reaction flask, protect with nitrogen, cool to -78°C, and stir for 30min. Slowly add 20 mL of 1.6M n-butyllithium hexane solution to the reaction flask dropwise using a syringe, and stir under this condition for 1 h after the dropwise addition is complete. A solution of tributyltin chloride in tetra...
Embodiment 2
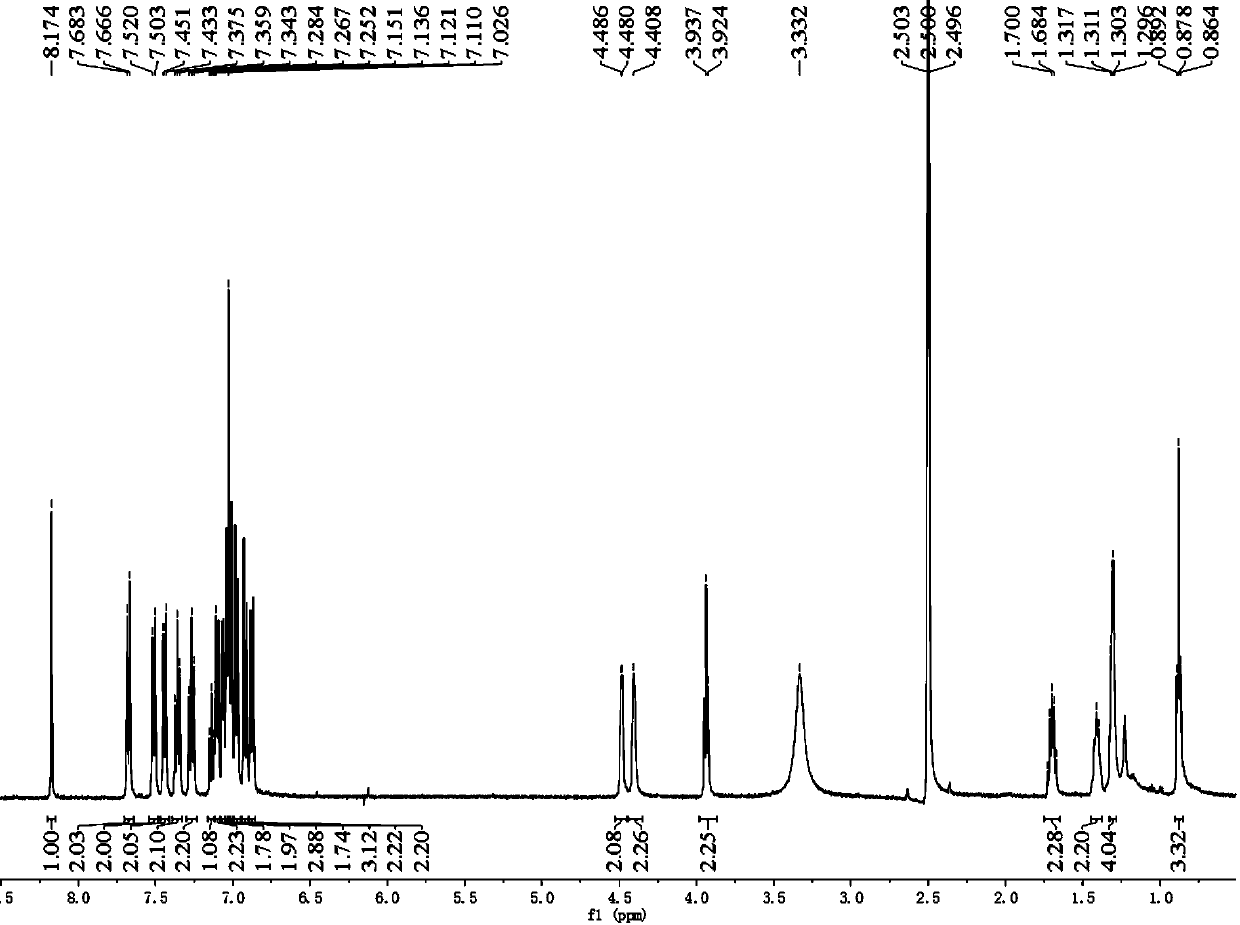
[0106] Example 2 Photosensitizing dye II (2-cyano-3-((2-(4-(2-(4-n-hexyloxytriphenylamine))vinyl)triphenylamine) 3,4-ethylenedioxythiophene) Acrylic acid) and preparation method thereof
[0107] The chemical structural formula of photosensitizing dye II is:
[0108] The synthetic route of photosensitive dye II is as follows:
[0109]
[0110] The preparation method of photosensitizing dye II is similar to that of Example 1, except that the secondary electron donor in Example 1 is Intermediate 5, and this Example is Intermediate 11; correspondingly, the intermediate in Example 1 In this embodiment, bodies 6-8 are replaced by intermediate body 12, intermediate body 13, and intermediate body 14, respectively.
[0111] Intermediate 11 was prepared from 4-hexyloxytriphenylamine via Vilsmeier reaction, sodium borohydride reduction and Michaelis–Arbuzov reaction.
[0112] The synthetic method of intermediate 11 is carried out in the order of following steps (21)-(23):
[011...
Embodiment 3
[0131] Example 3 Photosensitizing dye III (2-cyano-3-((2-(4-(4-(4-methoxytriphenylamine))butadienyl)triphenylamine)3,4-ethylenedioxy Thiophene) acrylic acid) and preparation method thereof
[0132] Photosensitizing dye III is a D-D-π-A structure with triphenylamine as a double electron donor, and its chemical structural formula is:
[0133] The synthetic route of photosensitive dye III is as follows:
[0134]
[0135] The preparation method of photosensitizing dye III is similar to Example 1, the difference is that in Example 1, the primary electron donor is Intermediate 2, and the secondary electron donor is Intermediate 5. In this example, the primary electron donor is Intermediate 5. The donor is intermediate 16, and the secondary electron donor is intermediate 18 instead. Correspondingly, the intermediates produced in the subsequent reaction in this embodiment are intermediate 19, intermediate 20, and intermediate 21.
[0136] The synthesis of the primary electron d...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com