A kind of anti-PD-L1 antibody and application, preparation method, kit and medicine
A PD-L1 and antibody technology, applied in biochemical equipment and methods, antibodies, drug combinations, etc., can solve the problems of difficult gene detection, inability to detect silencing or expression, increased workload, etc., and achieve high specificity and affinity Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0043] In another aspect, the present invention provides a method for preparing the above-mentioned anti-PD-L1 antibody, which comprises: immunizing animals with an antigenic peptide having an amino acid sequence as shown in positions 29-290 of SEQ ID No.2.
[0044] The present invention shows that the 29-290 position of PD-L1 has good antigenicity and contains B cell epitope through bioinformatics analysis, and the 29-290 region of PD-L1 is used as the recombinant protein (antigenic peptide) to immunize mice to prepare mouse monoclonal Anti-PD-L1 antibody, the obtained anti-PD-L1 antibody can recognize PD-L1 and has better specificity.
[0045] Of course, it should be noted that other animals such as rabbits, rats, pigs and other mammals can also be selected for immunization to prepare antibodies.
[0046] Further, in some embodiments of the present invention, before immunization, the preparation method further includes: transforming E. coli with an expression vector containi...
Embodiment 1
[0055] Construction of PD-L1 recombinant protein expression plasmid
[0056] According to the nucleic acid sequence in Genebank (GI: 20070268) and the encoded protein sequence of Q9NZQ7, the PD-L1 monoclonal is synthesized by gene synthesis corresponding to the amino acid sequence from the 29th to the 290th position of the PD-L1 protein (SEQ ID NO.2). The DNA sequence ((SEQ ID NO.1) was introduced with restriction sites EcoR I and Xho I, cloned into the expression vector pET41a (Novagen Company), and sequenced and analyzed, and the sequenced correct clones were selected for protein expression and purification.
Embodiment 2
[0058] PD-L1 recombinant protein expression and purification
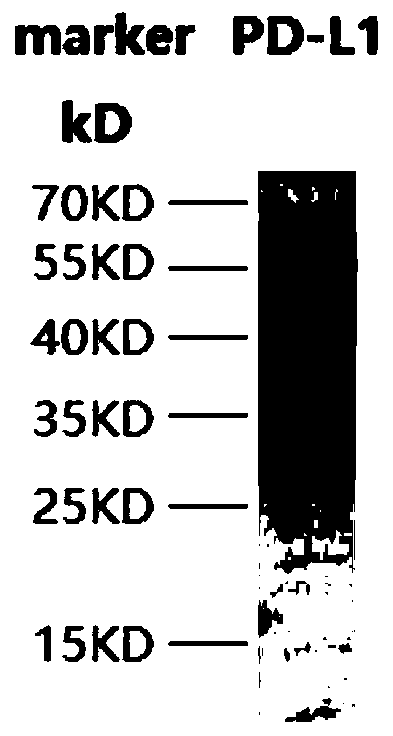
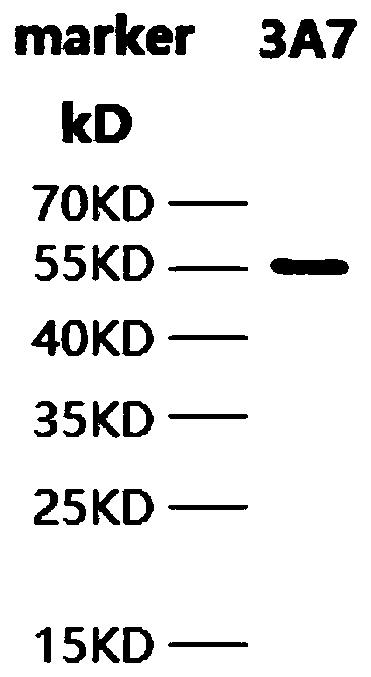
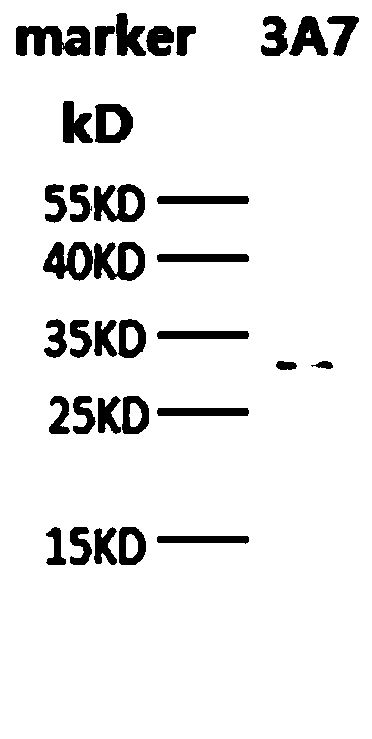
[0059] Escherichia coli containing the plasmid with the correct sequence of PD-L1 was cultivated to OD 600 0.5, add 10 μmol / L IPTG, culture overnight at 16°C, and after harvesting, sonicate and carry out Ni2 + Affinity purification. And carry out 12% SDS-PAGE detection, Coomassie brilliant blue staining, the result is as follows figure 1 As shown, the recombinant PD-L1 protein molecular weight is about 57kDa,
[0060] figure 1 The result (in the figure: marker is the molecular weight marker) shows that there is a band at the position of 55KD, indicating that the expression of PD-L1 recombinant protein obtained in this example is correct.
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com