Electrochemical method for synthesizing acidic hydrogen peroxide
A hydrogen peroxide, electrochemical technology, applied in the direction of electrodes, electrolysis process, electrolysis components, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
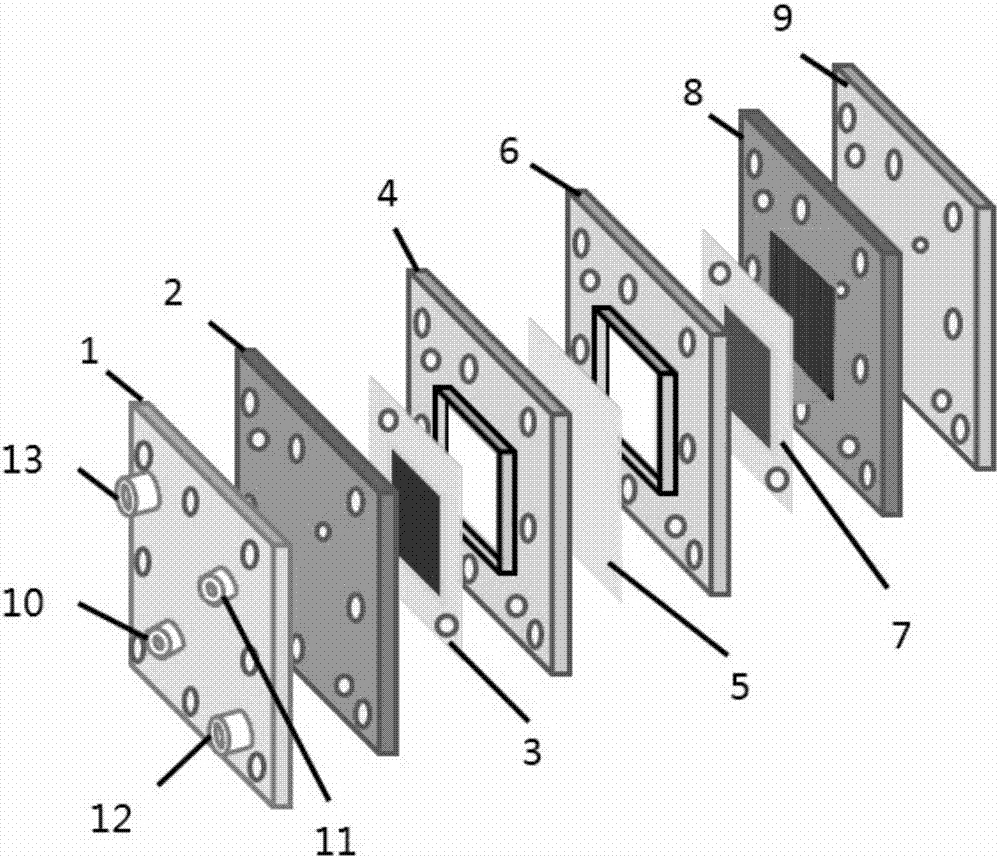
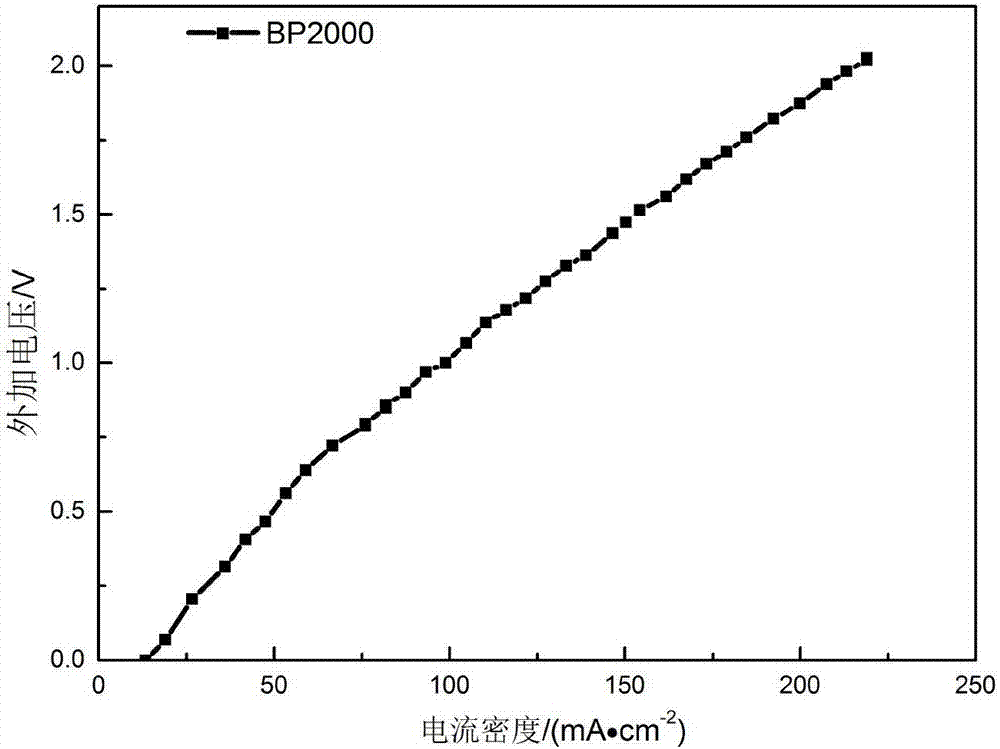
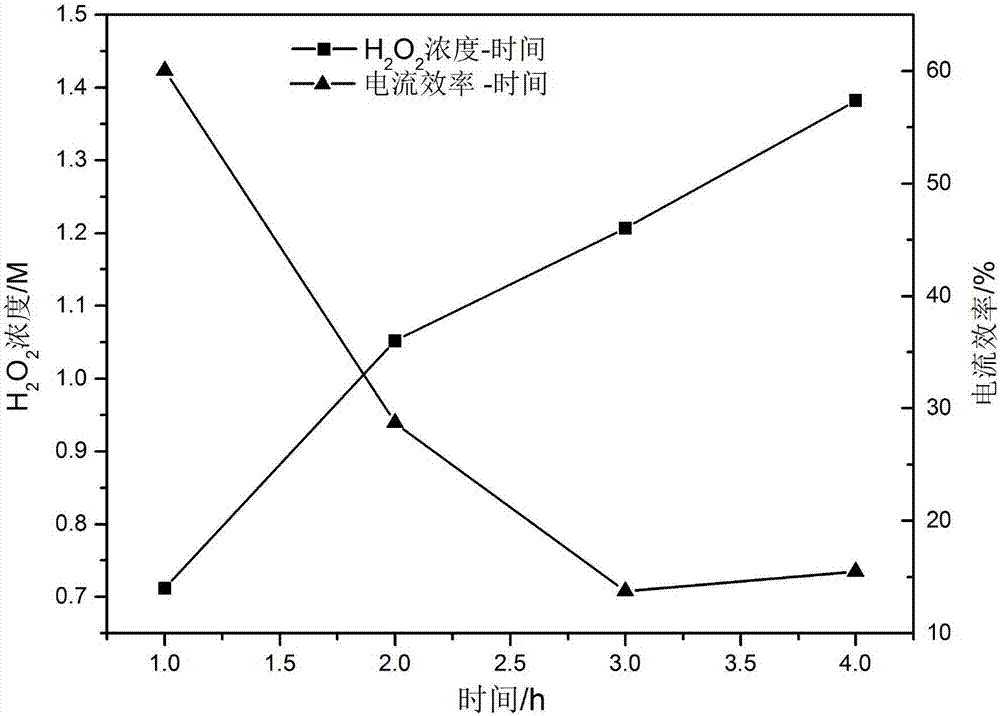
[0035] Using BP2000 as the cathode catalyst, the catalyst loading is 2mg / cm 2 ; Pt / C is the anode catalyst, the catalyst loading is 0.6mg / cm 2 ;The loading capacity of PTFE in the carbon paper and the catalytic layer is 40% and 20% respectively; the operating temperature is 25°C, the graphite engraved with the flow field is the cathode and anode end plates, the anode hydrogen pressure is 0.1MPa, and the flow rate is 64mL / min ; The oxygen pressure into the cathode is 0.1MPa, the flow rate is 76mL / min; the electrolyte is 0.5M H 2 SO 4 Solution, electrolyte circulation, flow rate is 10mL / min. After the external circuit is closed, a voltage of 1.5V is applied to both ends of the reactor through a constant current power supply. Under this condition, the current density is 144mA / cm 2 , after electrolysis for 2h, the concentration of hydrogen peroxide was 4%.
Embodiment 2
[0037] Using BP2000 as the cathode catalyst, the catalyst loading is 2mg / cm 2 ; Pt / C is the anode catalyst, the catalyst loading is 0.6mg / cm 2 ;The loading capacity of PTFE in the carbon paper and the catalytic layer is 40% and 20% respectively; the operating temperature is 25°C, the graphite engraved with the flow field is the cathode and anode end plates, the anode hydrogen pressure is 0.1MPa, and the flow rate is 64mL / min ; The oxygen pressure into the cathode is 0.1MPa, the flow rate is 76mL / min; the electrolyte is 0.5mM H 2 SO 4 , electrolyte circulation, flow rate of 10mL / min. After the external circuit is closed, a voltage of 1.5V is applied to both ends of the reactor through a constant current power supply. Under this condition, the current density is 7mA / cm 2 , after electrolysis for 2h, the concentration of hydrogen peroxide was 0.034%.
Embodiment 3
[0039] Using BP2000 as the cathode catalyst, the catalyst loading is 2mg / cm 2 ; Pt / C is the anode catalyst, the catalyst loading is 0.6mg / cm 2 ;The loading capacity of PTFE in the carbon paper and the catalytic layer is 40% and 20% respectively; the operating temperature is 25°C, the graphite engraved with the flow field is the cathode and anode end plates, the anode hydrogen pressure is 0.1MPa, and the flow rate is 64mL / min ; The oxygen pressure into the cathode is 0.1MPa, the flow rate is 76mL / min; the electrolyte is 0.5M H 2 SO 4 Solution, electrolyte circulation, flow rate is 10mL / min. After the external circuit is closed, a voltage of 0.5V is applied to both ends of the reactor through a constant current power supply. Under this condition, the current density is 47mA / cm 2 , After electrolysis for 2h, the concentration of hydrogen peroxide was 1.89%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com