Aqueous electrolyte and aqueous metal ion battery
A water-based electrolyte and electrolyte technology, applied in aqueous electrolytes, secondary batteries, circuits, etc., can solve the problems of low capacity retention or coulombic efficiency, material dissolution, etc., and achieve high coulombic efficiency, capacity retention, and coulombic efficiency The effect of high, high conductivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] 1.44g of water and 11.12g of LiTFSI were mixed and stirred to obtain an aqueous electrolyte. After testing, the conductivity of the aqueous electrolyte is 10 mS / cm.
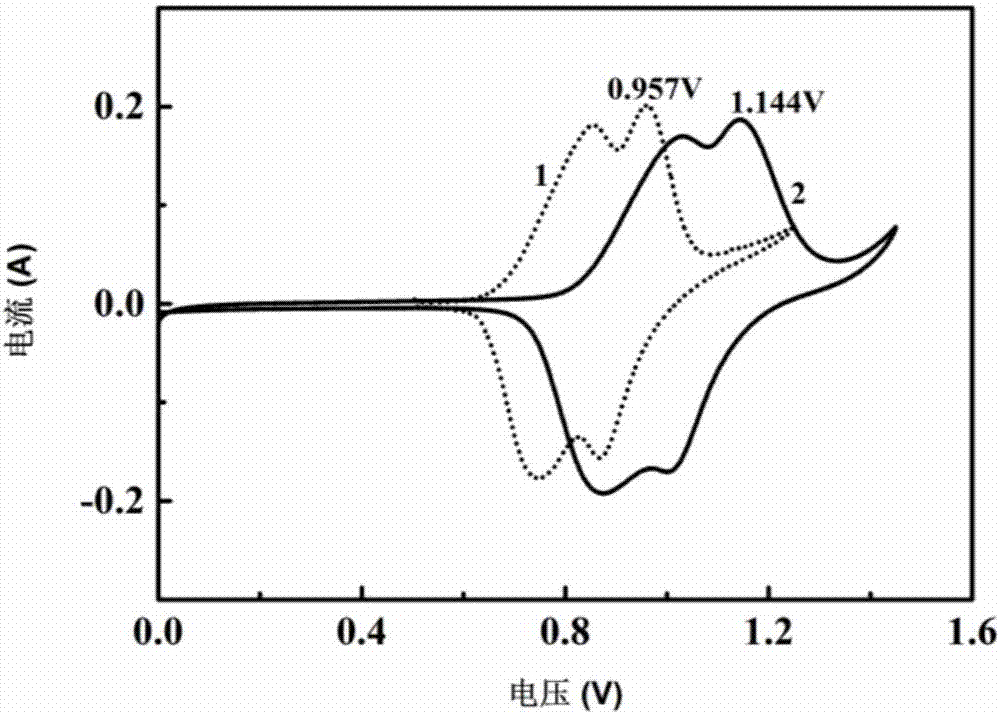
[0040] The aqueous electrolyte described above is used as the electrolyte, and a three-electrode system is adopted. Li 2 MnO 4 As the working electrode, the Ti sheet is the counter electrode, and Ag / AgCl (saturated KCl) is the reference electrode. Among them, the electrode slurry is prepared according to m (active material): m (polyvinylidene fluoride, PVDF): m (acetylene black) = 75:10:15, and the prepared slurry is coated on the Ti grid, and the positive electrode The mass is about 5-8 mg, and the mass of the negative electrode is 30%-40% more than that of the positive electrode. Put the coated pole pieces into an oven at 80°C, and bake in an air atmosphere for 13 hours. According to the above manufacturing method, the prepared pole piece is assembled into a half battery, and then a full battery (aqu...
Embodiment 2
[0044] Mix 7.12g of G3 with 1.44g of water, then add 11.12g of LiTFSI, stir to obtain an aqueous electrolyte. After testing, the conductivity of the aqueous electrolyte is 3.18mS / cm.
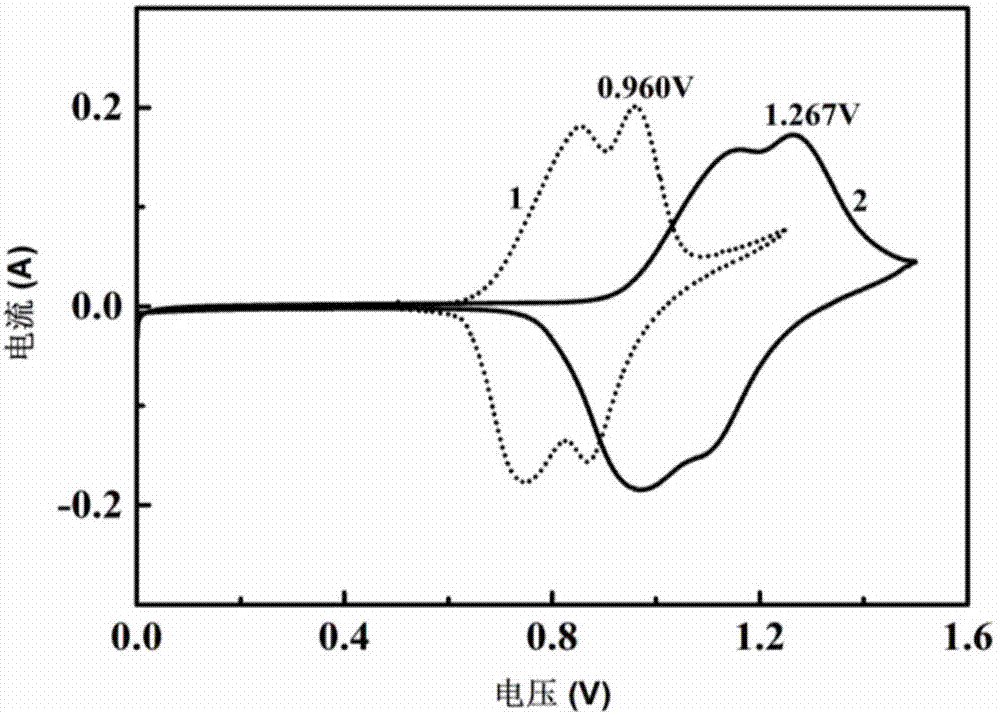
[0045] The aqueous electrolyte described above is used as the electrolyte, and a three-electrode system is adopted. Li 2 MnO 4 As the working electrode, the Ti sheet is the counter electrode, and Ag / AgCl (saturated KCl) is the reference electrode. Among them, the electrode slurry is prepared according to m (active material): m (polyvinylidene fluoride, PVDF): m (acetylene black) = 75:10:15, and the prepared slurry is coated on the Ti grid, and the positive electrode The mass is about 5-8 mg, and the mass of the negative electrode is 30%-40% more than that of the positive electrode. Put the coated pole pieces into an oven at 80°C, and bake in an air atmosphere for 13 hours. According to the above manufacturing method, the prepared pole piece is assembled into a half battery, and then a full b...
Embodiment 3
[0049] Mix 8.92g of G4 with 1.44g of water, then add 11.12g of LiTFSI, and stir to obtain an aqueous electrolyte. After testing, the conductivity of the aqueous electrolyte is 2.62mS / cm.
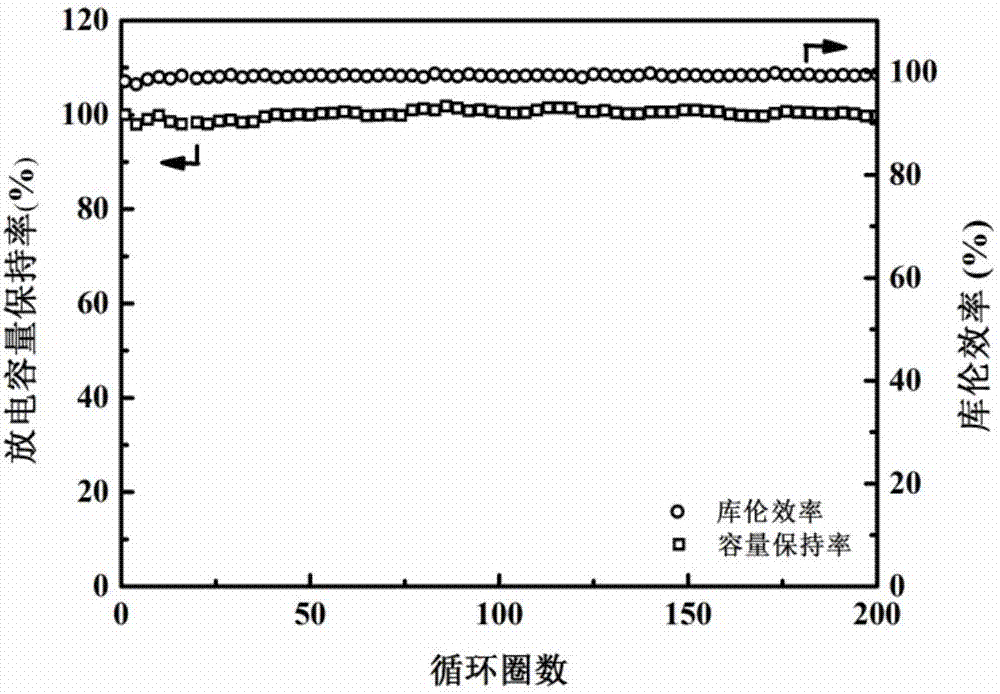
[0050] The aqueous electrolyte described above is used as the electrolyte, and a three-electrode system is adopted. Li 2 MnO 4 As the working electrode, the Ti sheet is the counter electrode, and Ag / AgCl (saturated KCl) is the reference electrode. Among them, the electrode slurry is prepared according to m (active material): m (polyvinylidene fluoride, PVDF): m (acetylene black) = 75:10:15, and the prepared slurry is coated on the Ti grid, and the positive electrode The mass is about 5-8 mg, and the mass of the negative electrode is 30%-40% more than that of the positive electrode. Put the coated pole pieces into an oven at 80°C, and bake in an air atmosphere for 13 hours. According to the above manufacturing method, the prepared pole piece is assembled into a half battery, and then a fu...
PUM
| Property | Measurement | Unit |
|---|---|---|
| electrical conductivity | aaaaa | aaaaa |
| electrical conductivity | aaaaa | aaaaa |
| electrical conductivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com