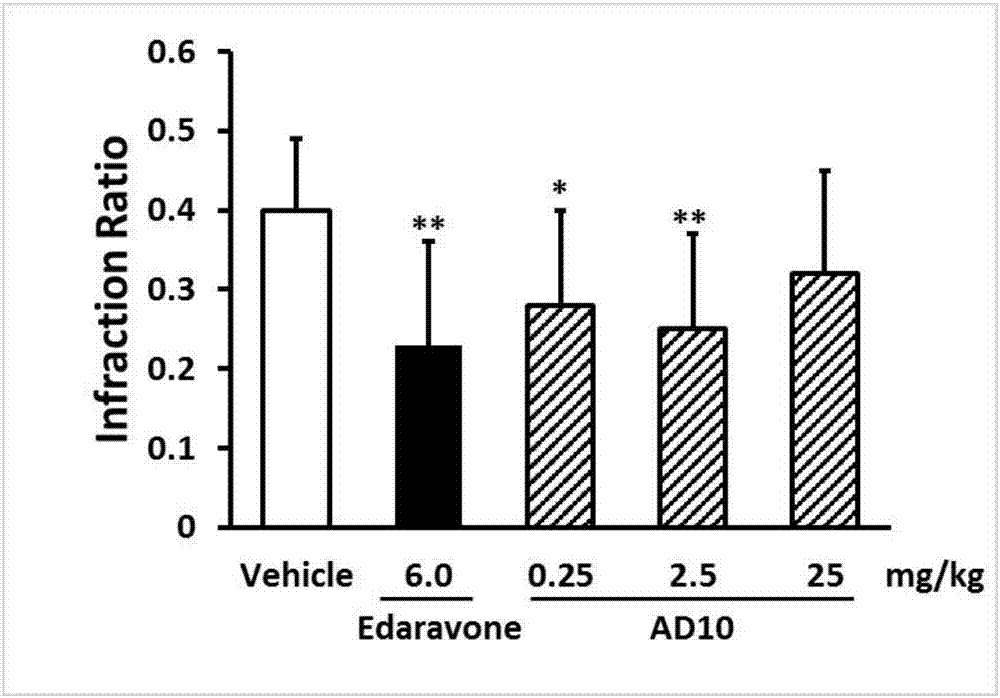
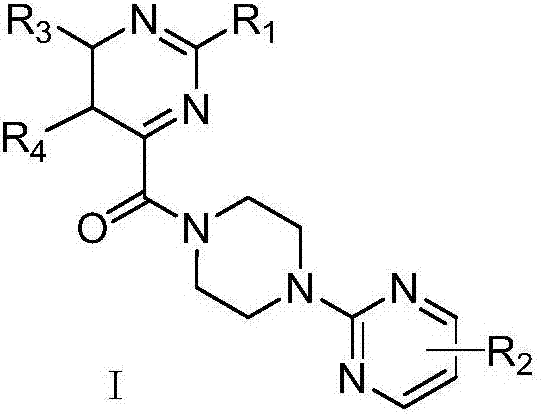
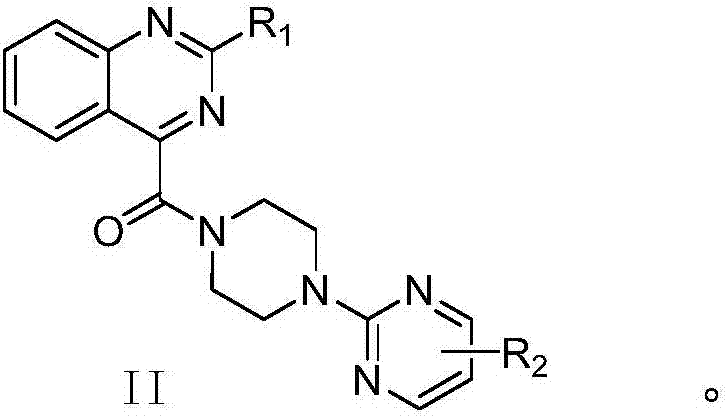
Pyrimidinylpyrazinamide compound, and application thereof
A technology of pyrimidine piperazine amide and pyrimidine piperazine, which is applied in the field of medicinal chemistry, can solve problems such as the lack of effective treatment drugs, and achieve the effects of reducing the size of cerebral infarction, easily obtaining raw materials and having strong inhibitory effect.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0066] The preparation of embodiment 1 (4-(2-pyrimidine piperazinyl)) (2-chloroquinazoline) ketone (compound 10)
[0067]
[0068] Add 100g of indolinone (isatin) and a stirrer into a 500ml eggplant-shaped bottle, prepare an aqueous solution of 1.5 equivalents of KOH according to 20% (w / w), add in batches, after the addition, heat to 40°C, the red indole The ketone solution gradually becomes shallow as the reaction progresses, and the reaction is completed after 8 hours. The water is evaporated under reduced pressure below 40°C, and a small amount of ethanol is added to the solid initial product. After uniform suspension, filter, wash twice with a small amount of ethanol, remove KOH, and Wash with acetone twice to remove ethanol and water. To avoid moisture absorption, the yellow filter cake was quickly sealed and dried in vacuum. Mass spectrometry identification (ESI-MS calcd.for C 8 h 6 KNO 3 : [M+H]+, 204.24; found, 204.00) The crude product is compound 6, a yellow p...
Embodiment 2
[0072] Preparation of Example 2 (4-(2-pyrimidine piperazinyl)) (2-cyanoquinazoline) ketone (10a)
[0073]
[0074] 250mg compound 10 was dissolved in DMSO:H with 1.2 equivalents of sodium cyanide and 1 equivalent of 1,4-diazabicyclo[2.2.2]octane (DABCO) 2 In the mixed solution of O=9:1, under the protection of nitrogen, seal and heat to 100°C, and keep stirring. After 5 hours, the reaction was completed, cooled to room temperature, added 300ml of water, extracted 4 times with 50ml of dichloromethane, washed the combined dichloromethane layer once with 100ml water, once with 100ml saturated saline, dried over anhydrous sodium sulfate, and evaporated to dryness Silica gel column chromatography gave the yellow target product 10a in a yield of 70%.
[0075] 1 H NMR (400M Hz, CDCl 3 ): δ8.35(d, J=4.4Hz, 2H), 8.21(d, J=8.0Hz, 1H), 8.13-8.09(m, 2H), 7.87(t, J=7.6Hz, 1H), 6.58 (t,J=4.8Hz,1H),4.08-4.06(m,2H),4.03-4.00(m,2H),3.89-3.86(m,2H),3.38-3.36(m,2H); 13 C NMR (100MHz, CDC...
Embodiment 3
[0076] Preparation of Example 3 (4-(2-pyrimidinepiperazinyl)) (2-N, N-dimethylaminoquinazoline) ketone (10b)
[0077]
[0078] Dissolve 350mg of compound 10, 2 equivalents of dimethylamine hydrochloride and 2 equivalents of 1,8-diazabicycloundec-7-ene (DBU) in 5ml of methanol, keep stirring under nitrogen protection, seal the tube and heat to 80°C After 12 hours, the reaction was completed, the heating was stopped, cooled to room temperature, the reaction liquid was directly evaporated to dryness, and silica gel column chromatography gave the yellow-green target product 10b with a yield of 48%.
[0079] 1 H NMR (400M Hz, CDCl 3 ): δ8.31(d, J=8.0Hz, 2H), 7.69(d, J=8.8Hz, 1H), 7.66-7.56(m, 2H), 7.15(t, J=7.6Hz, 1H), 6.53 (t,J=4.8Hz,1H),4.03-4.01(m,2H),3.98-3.96(m,2H),3.81-3.79(m,2H),3.37-3.35(m,2H),3.28(s ,6H); 13 C NMR (100MHz, CDCl 3 ): δ165.4, 163.4, 161.5, 158.8, 157.8(2C), 153.6, 134.4, 126.1, 125.5, 122.5, 115.4, 110.6, 46.5, 44.0, 43.6, 41.6, 37.2(2C); ESI-MS calc...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com