Synthesis method of isocytosine
A technique for the synthesis of isocytosine, which is applied in the field of chemical synthesis, can solve problems such as environmental hazards, failure to achieve, and unsatisfactory yields, and achieve the effects of wide sources, reduced by-product formation, and increased yields
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] The synthesis of embodiment one isocytosine
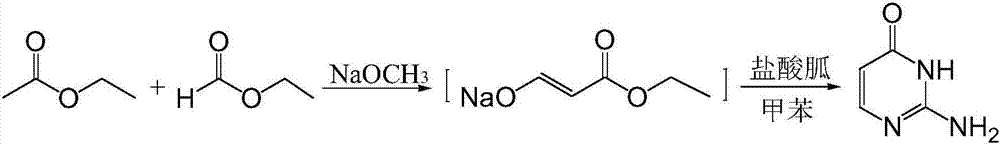
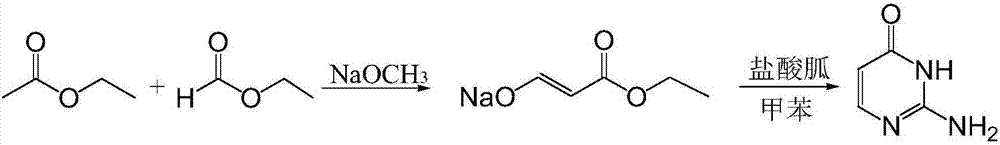
[0027] Step a), the synthesis of ethyl hydroxyacrylate sodium salt, the synthetic formula is:
[0028]
[0029] Put 10.5g (0.194mol) of catalyst sodium methoxide and 10.5ml (0.13mol) of ethyl formate as reactant into the reaction kettle in sequence at -5~0°C. ml (0.14mol) was slowly added dropwise into the reactor, refluxed for 4 hours, and cooled to room temperature to obtain the intermediate sodium salt of ethyl hydroxyacrylate.
[0030] Step b), the synthesis of isocytosine, the synthetic formula is:
[0031]
[0032] Add an appropriate amount of methanol to the sodium salt of the intermediate ethyl hydroxyacrylate prepared in the previous step to dissolve it just enough, add catalyst 3A molecular sieve, add 8.7g (0.09mol) of guanidine hydrochloride, heat to 90°C and reflux for 3h, cool to room temperature, and evaporate the mixture to dryness Add an appropriate amount of pure water until the mixture is just disso...
Embodiment 2
[0036] The synthesis of embodiment two isocytosine
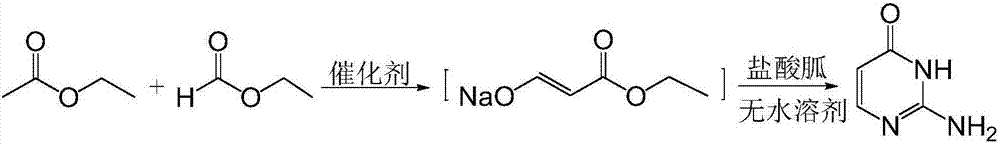
[0037] Step a), the synthesis of ethyl hydroxyacrylate sodium salt, the synthetic formula is:
[0038]
[0039] Put 3.45g (0.15mol) of catalyst sodium and 8.67ml (0.12mol) of ethyl formate reactant into the reaction kettle at -5~0°C in sequence, after stirring evenly, heat to 75°C and keep it under reflux, and add 14.6ml of ethyl acetate (0.15mol) was slowly added dropwise into the reactor, refluxed for 4h, and cooled to room temperature to obtain the intermediate sodium salt of ethyl hydroxyacrylate.
[0040] Step b), the synthesis of isocytosine, the synthetic formula is:
[0041]
[0042] Add an appropriate amount of n-butanol to the sodium salt of the intermediate hydroxyethyl acrylate prepared in the previous step to dissolve it just enough, add catalyst 3A molecular sieve, put in 11.46 g (0.12 mol) of guanidine hydrochloride, heat to 90 ° C and reflux for 3 h, cool to room temperature, evaporate The residual so...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com