Monoclonal cell strain and application of monoclonal cell strain for measuring relative biological activity of IL-6R inhibitors
A technology of biological activity and cell line, applied in the field of measuring the relative biological activity of IL-6R inhibitors, monoclonal cell line and its construction, can solve the problems of unstable measurement results, high culture requirements, poor repeatability, etc. The effect of short assay cycle, simple cell culture, and low reagent requirements
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
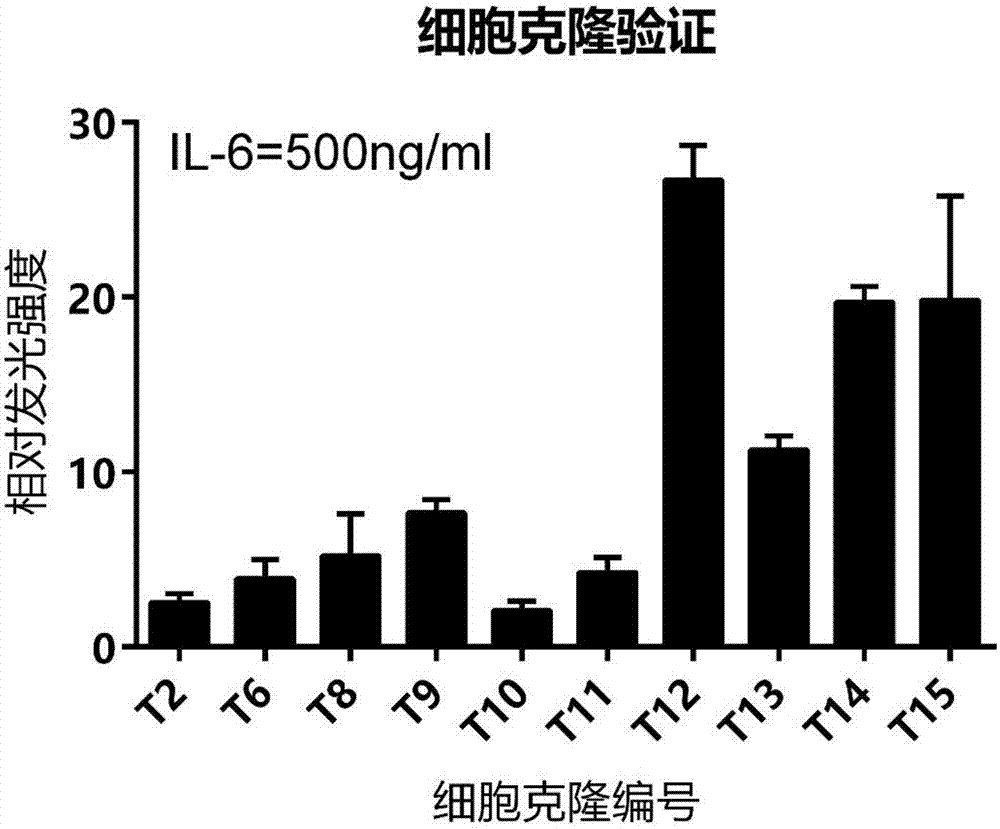
[0073] This embodiment is the construction of monoclonal cell line 293T-SIE, which includes the following steps:
[0074] (1) Determination of the optimal screening concentration of hygromycin b;
[0075] 293T cells were cultured in DMEM medium containing 10% fetal bovine serum (FBS). Cells in the logarithmic growth phase were taken, and the cell concentration was adjusted to 1×10 6 / ml, 2ml per well was inoculated in a 6-well plate, and placed in a cell culture incubator for routine culture. After 24 hours of cell inoculation, dilute hygromycin b, establish a total of 10 concentration gradients of 0-1 mg / ml, add it to a 6-well plate, observe the growth of cells every day, and the lowest hygromycin that causes all cells to die within 10-14 days The b concentration is the working concentration for screening and transfecting 293T cells. In the human kidney epithelial cell line 293T-SIE stably expressing luciferase regulated by SIE, the optimal working concentration of hygromy...
Embodiment 2
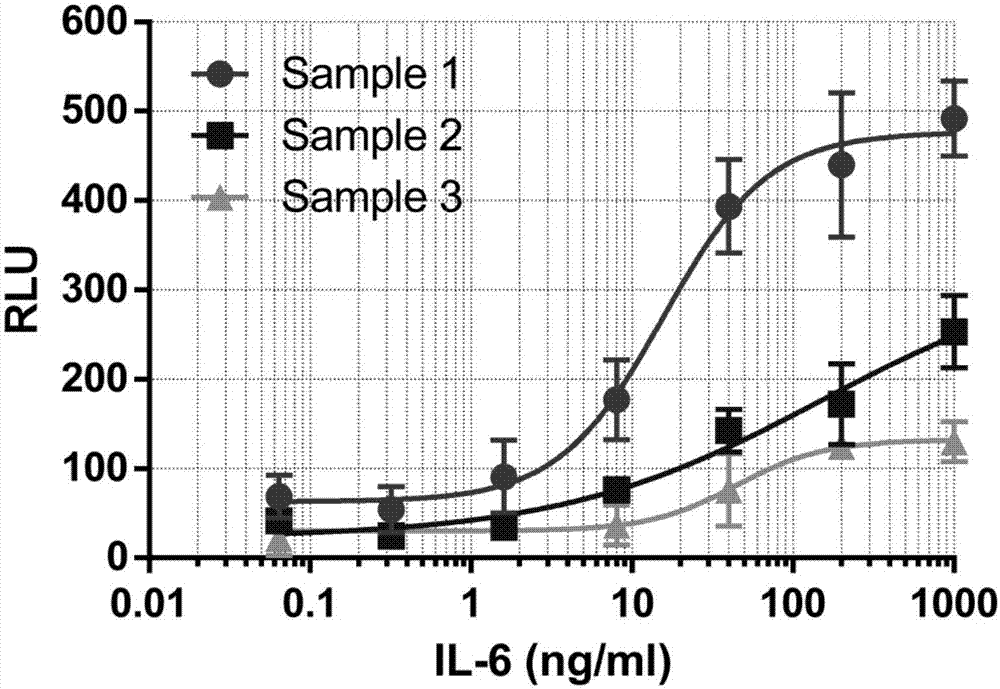
[0087] This embodiment is the condition optimization of the luciferase reporter gene method for determining the relative biological activity of IL-6R inhibitors, which includes the following steps:
[0088] (1) Recovery and subculture of cells: Take out the frozen 293T-SIE cells from the liquid nitrogen tank, thaw the cells quickly, transfer them to 5ml DMEM medium containing 10% FBS, and store them at 37°C and 5% CO 2 cultured in an incubator.
[0089] (2) Cell inoculation: the cells in the logarithmic growth phase were digested with 0.25% trypsin, centrifuged at 1000rpm for 2min, the supernatant was discarded, and the cells were resuspended in the cell culture medium (containing 10% fetal bovine serum in phenol red-free DMEM) base), count. Dilute the cell suspension to 0.8×10 6 cells / ml, 0.4×10 6 cells / ml, 0.2×10 6 cells / ml, inoculated in 96-well white plate at 50 μl / well.
[0090](3) Add IL-6 gradient solution: take 100 μg of human recombinant IL-6 powder, add 1 ml of ...
Embodiment 3
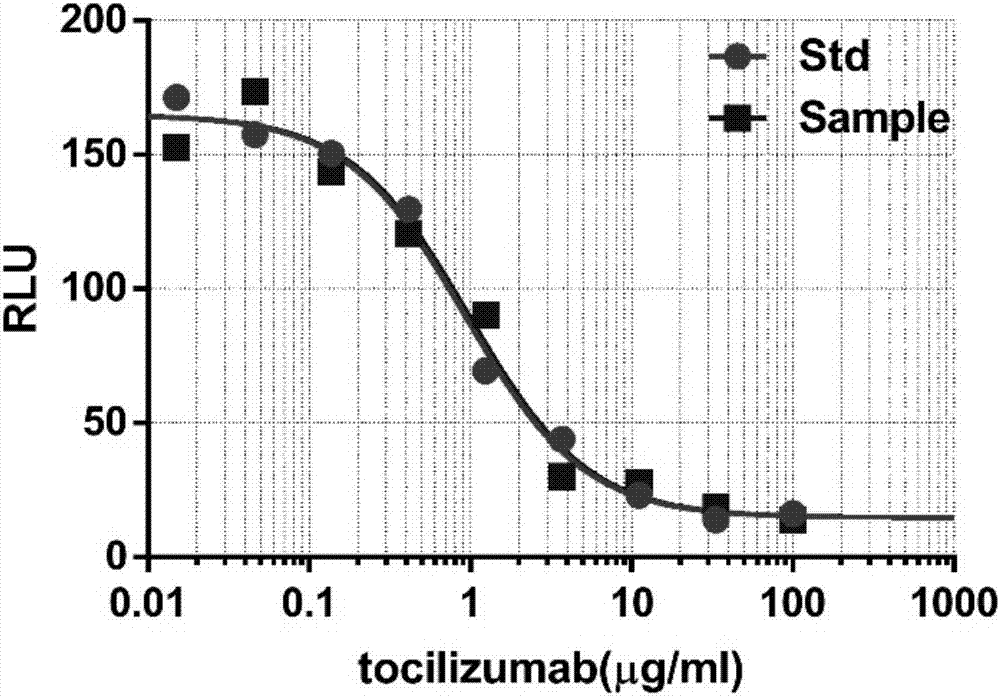
[0096] This example is a luciferase reporter gene method to determine the relative biological activity of tocilizumab.
[0097] The method for measuring the relative biological activity of the IL-6R inhibitor tocilizumab using 293T-SIE cell lines comprises the following steps:
[0098] (1) Recovery and subculture of cells: Take out the frozen 293T-SIE cells from the liquid nitrogen tank, thaw the cells quickly, transfer them to 5ml DMEM medium containing 10% FBS, and store them at 37°C and 5% CO 2 cultured in an incubator.
[0099] (2) Cell inoculation: the cells in the logarithmic growth phase were digested with 0.25% trypsin, centrifuged at 1000rpm for 2min, the supernatant was discarded, and the cells were resuspended in the cell culture medium (containing 10% fetal bovine serum in phenol red-free DMEM) base), count. Dilute the cell suspension to 0.8 x 10 6 After cells / ml, inoculate 96-well white plate with 50 μl / well.
[0100] (3) Preparation of IL-6 working solution: ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com