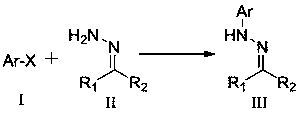
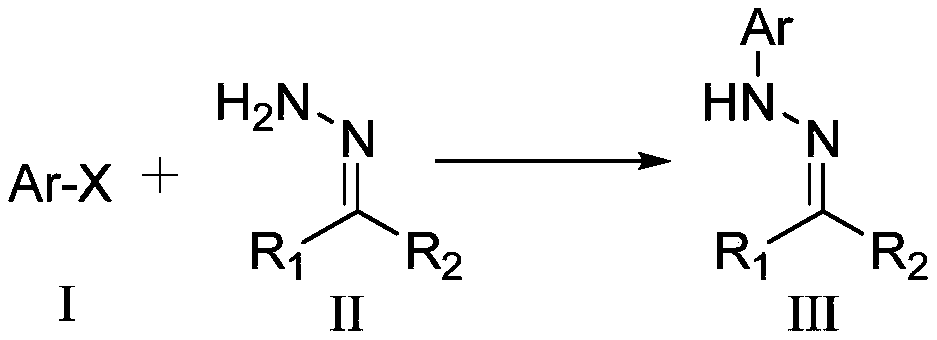
A method for synthesizing n-aryl hydrazones from halogenated aromatic hydrocarbons and hydrazone compounds under visible light catalysis
A halogenated aromatic hydrocarbon, visible light technology, applied in chemical instruments and methods, preparation of organic compounds, preparation of hydrazones, etc., can solve the problems of poor substrate applicability, expensive palladium, high synthesis cost, and achieve a wide range of substrates. , mild reaction conditions, high catalytic efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0018] Synthetic structural formula following compound (1)
[0019]
[0020] Benzophenone hydrazone (196mg, 1mmol), bromobenzene (62.8mg, 0.4mmol), multi-substituted BODIPY organic photocatalyst (3.4mg, 0.008mmol), NiBr 2 (1.8mg, 0.008mmol), N-methyldicyclohexylamine (210μL, 1mmol), DMF (3mL) were added to the Schlenk reaction tube, nitrogen protection, at 60 ℃ with four 4W LED lights Irradiate the reaction for 10 h, cool to room temperature after the reaction is over, extract the reaction solution with ethyl acetate (10 mL) and wash with saturated brine three times (10 mL×3), collect the organic phase and dry it with anhydrous sodium sulfate, filter, and use a rotary evaporator After concentration, a mixture of petroleum ether and ethyl acetate with a volume ratio of 200:1 was used as an eluent for column chromatography to obtain 102 mg of yellow oily compound (1), with a yield of 94%. The characterization data are: 1 H NMR (400MHz, CDCl 3 ):δ7.61-7.57(m,4H),7.55-7.50(m,...
Embodiment 2
[0024] In this example, BODIPY-1 in Example 1 was replaced with BODIPY-2 in an equimolar amount, and the other steps were the same as in Example 1 to obtain 89 mg of yellow oily compound (1), with a yield of 82%.
[0025]
Embodiment 3
[0027]In this example, BODIPY-1 in Example 1 was replaced with BODIPY-3 in an equimolar amount, and the other steps were the same as in Example 1 to obtain 82 mg of yellow oily compound (1), with a yield of 76%.
[0028]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com