Method for recycling mother liquor during production of iron phosphate
A production process, iron phosphate master technology, applied in chemical instruments and methods, phosphorus compounds, inorganic chemistry, etc., can solve environmental pollution, waste and other problems, reduce production costs, reduce investment and operating costs, and avoid waste of resources Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
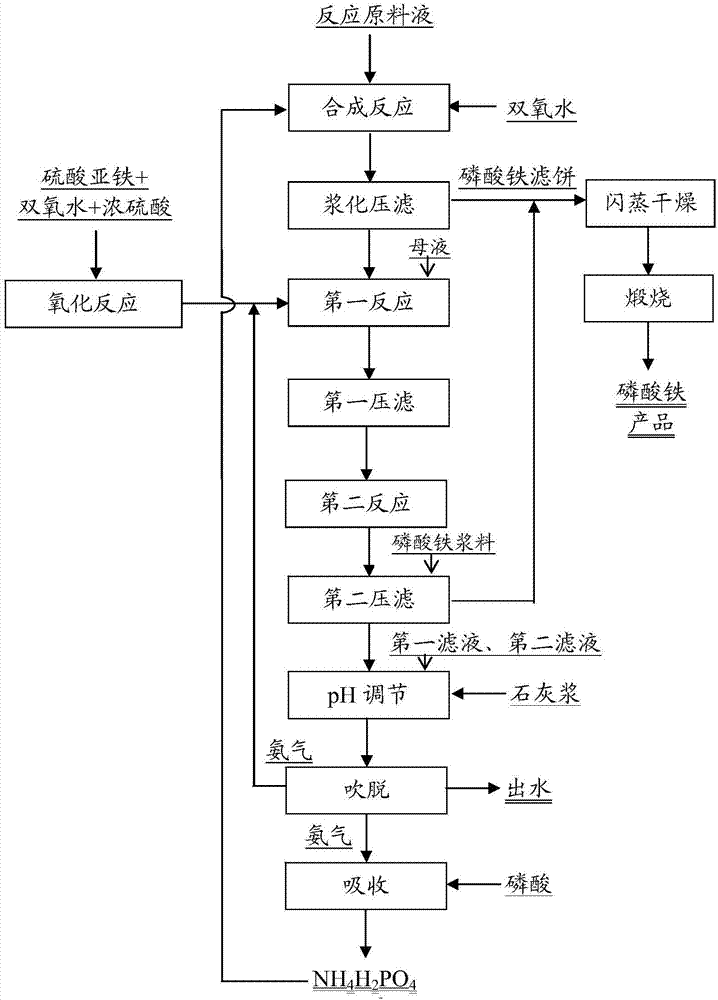
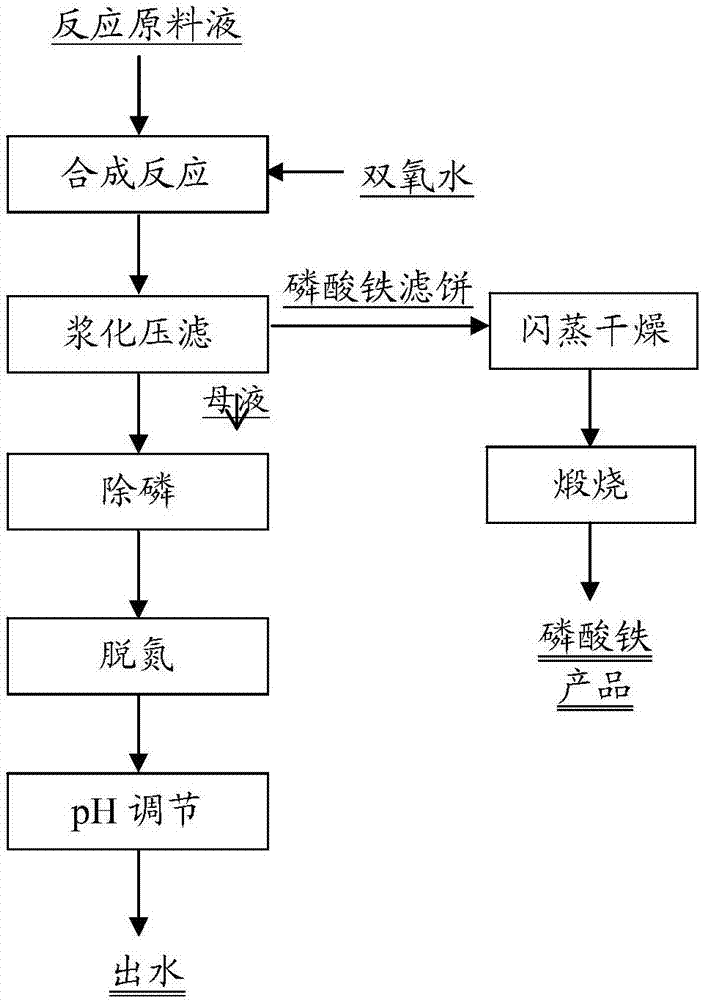
[0039] see figure 1 The process shown produces an iron phosphate product. The ferric phosphate reaction raw material solution and H 2 o 2 Add to the reaction kettle to carry out the synthesis reaction, and the raw material solution of the ferric phosphate reaction is made of NH 4 h 2 PO 4 , FeSO 4 、H 3 PO 4 Composition, the concentration of each component in the reaction raw material liquid is as follows: NH 4 h 2 PO 4 The mass concentration is 5.3%, FeSO 4 The mass concentration is 7.0%, H 3 PO 4 The mass concentration is 0.6%. H involved in the synthesis reaction 2 o 2 The mass concentration is 0.8%. The temperature of the synthesis reaction was controlled to be 80°C.
[0040] After slurry press filtration, the phosphorus concentration in the mother liquor was 9.8g / L, and the ammonia nitrogen concentration was 8g / L.
[0041] To FeSO with a volume of 50L and a concentration of 260g / L 4 Add volume 14.7L, concentration 27% H to the solution 2 o 2 And volume...
Embodiment 2
[0048] The difference with Example 1 is that the concentration of each component in the reaction raw material liquid is as follows: NH 4 h 2 PO 4 The mass concentration is 7.6%, FeSO 4 The mass concentration is 10.0%, H 3 PO 4 The mass concentration is 0.8%. H involved in the synthesis reaction 2 o 2 The mass concentration is 1.1%. The temperature of the synthesis reaction was controlled to be 90°C.
[0049] H in the oxidation reaction 2 o 2 with FeSO 4 The molar ratio is 1.2:1, and the pH of the first reaction is 3. During the stripping process, lime slurry was added to adjust the pH of the mixed filtrate to 11.
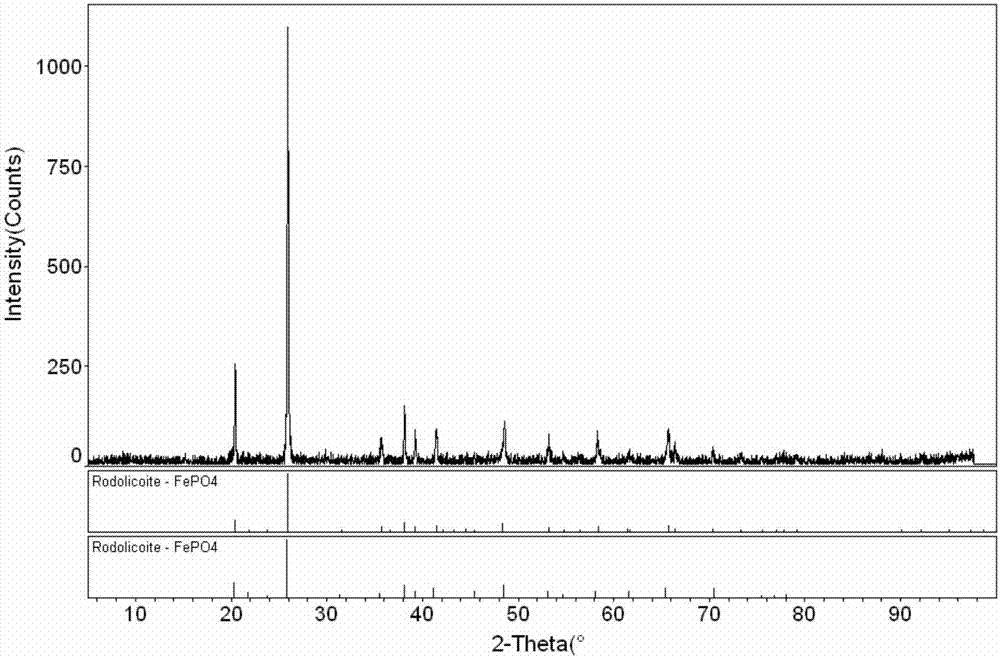
[0050] After flash drying and calcination, 1050g of qualified ferric phosphate product was prepared from 800L mother liquor, and the recovery rate of phosphorus was 95%; 4 h 2 PO 4 (converted into a dry weight of 48kg), the recovery rate of ammonia nitrogen reaches 90%, and the recovery and utilization of phosphorus and ammonium in the mother liquor a...
Embodiment 3
[0052] The difference from Example 1 is that in the oxidation reaction H 2 SO 4 with FeSO 4 The molar ratio is 1:20, the reaction temperature is 40°C, and the reaction time is 90min. Fe in the raw material liquid of the oxidation reaction and H in the mother liquid 3 PO 4 The molar ratio is 1.2:1, and the reaction pH is 4.
[0053] The first press filter cake, H 2 O and H 3 PO 4 The mass ratio of the second reaction is 0.5:10:0.33, and the second reaction temperature is 80°C. Use 2.5mol / L phosphoric acid to absorb the blown out ammonia gas.
[0054] After flash drying and calcination, 1060g of qualified ferric phosphate product was prepared from 800L mother liquor, and the recovery rate of phosphorus was 96%. 4 h 2 PO 4 (converted into dry weight 51kg), the recovery rate of ammonia nitrogen reaches 96%, and the recovery and utilization of phosphorus and ammonium in the mother liquor are realized.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com