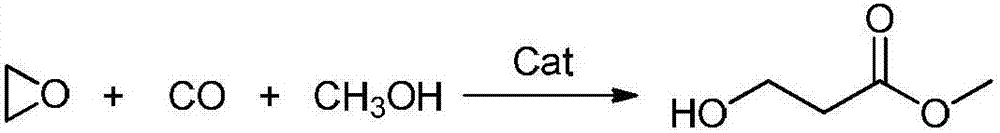
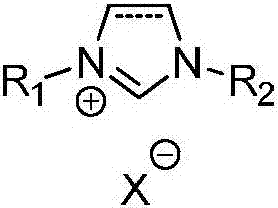
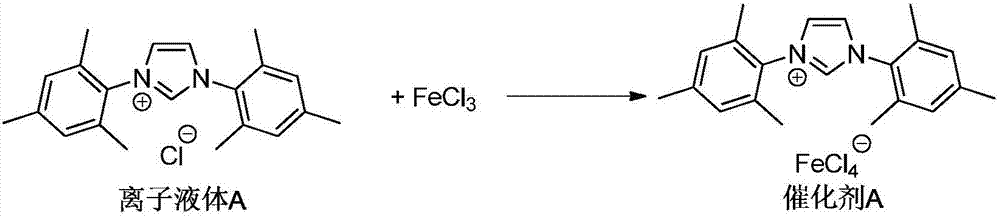Preparation method of methyl-3-hydroxypropanoate
A technology of methyl hydroxypropionate and methanol, which is applied in chemical instruments and methods, carbon monoxide or formate reaction preparation, organic compound/hydride/coordination complex catalyst, etc., can solve the problem of complex catalyst preparation and high energy consumption , the problem of high reaction temperature, to achieve the effect of low price, improved conversion rate and high activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Add 5 mmol of ionic liquid A, 5 mmol of ferric chloride, and 40 mL of acetone into a 100 mL reaction tube, and react at room temperature for 12 hours. After the reaction was completed, the solvent acetone was removed under reduced pressure, and catalyst A was obtained after vacuum drying.
[0034]
[0035] Add 1 mmol of Catalyst A to a 100 mL reactor, purged the reactor three times with nitrogen, added 10 mmol of ethylene oxide and 30 mL of methanol, introduced carbon monoxide to make the system pressure 5.0 MPa, and reacted at 60°C for 3 hours. After the reaction was completed, the reactor body was fully cooled to 0°C, and the pressure was slowly released to normal pressure. The reactor was purged three times with nitrogen, and samples were taken for analysis. The experimental results are shown in Table 1.
Embodiment 2
[0037] Add 5 mmol of ionic liquid B, 5 mmol of ferric chloride, and 40 mL of tetrahydrofuran into a 100 mL reaction tube, and react at room temperature for 12 hours. After the reaction was completed, the solvent tetrahydrofuran was removed under reduced pressure, and catalyst B was obtained after vacuum drying.
[0038]
[0039] Add 1mmol of Catalyst B to a 100mL reactor, purge the reactor with nitrogen three times, add 20mmol of ethylene oxide and 30mL of methanol, and introduce carbon monoxide to make the system pressure 8.0MPa, and react at 45°C for 8 hours. After the reaction was completed, the reactor body was fully cooled to 0°C, and the pressure was slowly released to normal pressure. The reactor was purged three times with nitrogen, and samples were taken for analysis. The experimental results are shown in Table 1.
Embodiment 3
[0041] Add 5 mmol of ionic liquid C, 5 mmol of ferric chloride, and 40 mL of acetone into a 100 mL reaction tube, and react at room temperature for 12 hours. After the reaction was completed, the solvent acetone was removed under reduced pressure, and catalyst C was obtained after vacuum drying.
[0042]
[0043] Add 1mmol of Catalyst C to a 100mL reactor, purge the reactor with nitrogen three times, add 30mmol of ethylene oxide and 30mL of methanol, feed in carbon monoxide, make the system pressure 3.0MPa, and react at 100°C for 6 hours. After the reaction was completed, the reactor body was fully cooled to 0°C, and the pressure was slowly released to normal pressure. The reactor was purged three times with nitrogen, and samples were taken for analysis. The experimental results are shown in Table 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com