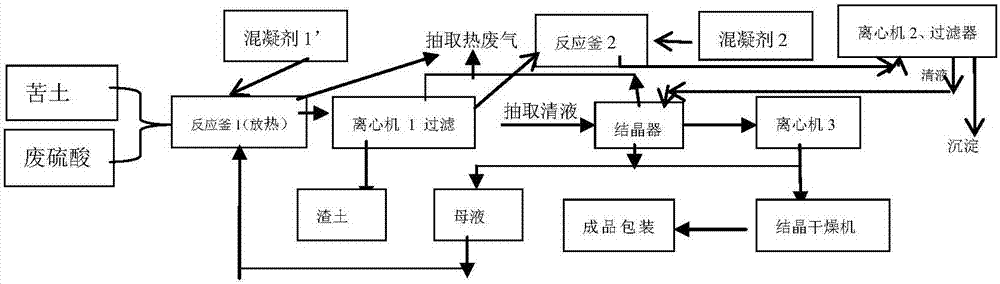
Method for producing magnesium sulfate from colored waste sulfuric acid in ion exchange resin production
An ion exchange resin, waste sulfuric acid technology, applied in magnesium sulfate, chemical instruments and methods, multi-stage water treatment, etc., can solve problems such as ineffective removal, complicated processes and equipment, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
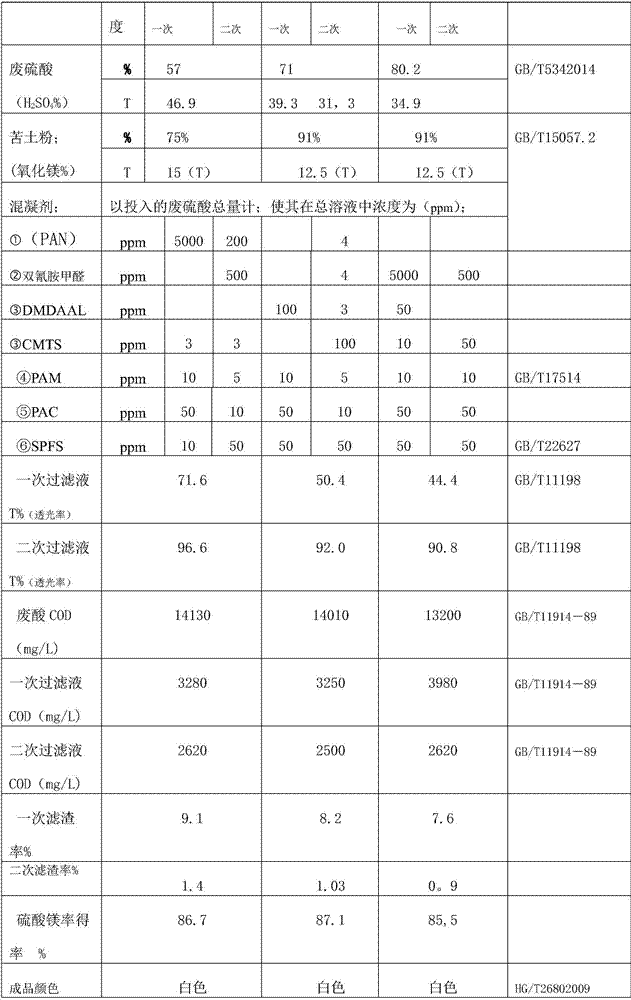
[0030] ① Take bitter soil powder (magnesia content 75%) and waste sulfuric acid (concentration 57%) as raw materials, and weigh them in equimolar amounts (1:1) according to the size of the reaction vessel; 15 tons of bitter soil powder. 46.9 tons of waste sulfuric acid.
[0031] ② Add waste sulfuric acid to the reaction kettle first, and under agitation, add coagulant (based on the total amount of waste sulfuric acid) by weighing respectively, so that the concentration in the total solution is: dodecyl dimethyl benzyl chloride Ammonium chloride (PAN) 5000ppm, carboxymethyl chitosan (CMTS) 3ppm, polyacrylamide 10ppm, polyaluminum chloride 50ppm, polyferric sulfate 10ppm; stir the solution evenly.
[0032] ③ When the alum flower and color of the solution remain unchanged, stand still for about half an hour, remove the precipitate through a cloth bag centrifuge or plate filter (filter for the first time), and put the filtrate (measured T%, COD) into the reaction kettle again.
...
Embodiment 2
[0037] ①With bitter soil powder (magnesia content 91%) and waste sulfuric acid (concentration 71%) as raw materials, the two are based on the size of the reaction vessel (50m 3 Reactor), the present embodiment feeds total volume by 40m 3 count. Equimolar amount (1:1) weighed; Bitter soil powder 12.5 tons. 39.3 tons of waste sulfuric acid.
[0038] ② The reaction kettle is first added with waste sulfuric acid, and under stirring, add coagulants (in terms of the total amount of waste sulfuric acid) by weighing respectively, so that the concentration in the total solution is: dimethyldienylpropyl ammonium chloride ( DMDAAL) 100ppm, carboxymethyl chitosan (CMTS) 10ppm, polyacrylamide 10PPm, polyaluminum chloride 50ppm, polyferric sulfate 50ppm (adding for the first time), stir the solution evenly.
[0039] ③ When the alum flowers and color of the solution are observed, stand still for about half an hour, remove the precipitate through a cloth bag centrifuge or plate filter (pri...
Embodiment 3
[0044] ① Take bitter soil powder (magnesia content 91%) and waste sulfuric acid with a concentration of 80.2% as raw materials, both of which are based on the size of the reaction vessel (50m 3 Reactor), the present embodiment feeds total volume by 40m 3 Equimolar amount (1:1) weighed; 12.5 tons of bitter soil powder. 34.9 tons of waste sulfuric acid.
[0045] ② The reaction kettle is first filled with waste sulfuric acid, and in the stirring state, the coagulant (based on the total amount of waste sulfuric acid) is weighed and added respectively, so that the concentration in the total solution is: dicyandiamide formaldehyde 5000ppm, dimethyl dienyl Propyl ammonium chloride (DMDAAL) 50ppm, carboxymethyl chitosan (CMTS) 10ppm, polyacrylamide 10ppm, polyaluminum chloride 50ppm, polyferric sulfate 50ppm.
[0046] ③ When the alum flowers and color of the solution are observed, stand still for about half an hour, and remove the precipitate through a cloth bag centrifuge or a plat...
PUM
| Property | Measurement | Unit |
|---|---|---|
| transmittivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com