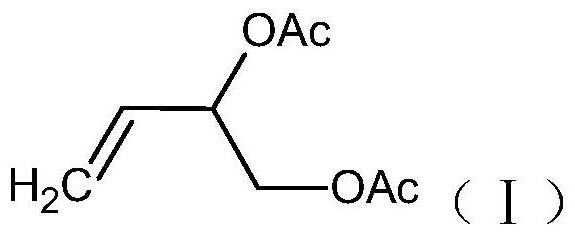
A kind of preparation method of 3,4-diacetoxy-1-butene
A technology of diacetoxy and butene, which is applied in the field of preparation of 3,4-diacetoxy-1-butene, can solve the problems of expensive catalyst, high catalyst consumption and low isomerization yield, etc. Achieve the effects of increased yield, simple and easy-to-obtain raw materials, and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0034] A kind of preparation method of 3,4-diacetoxy-1-butene, comprises the following steps:
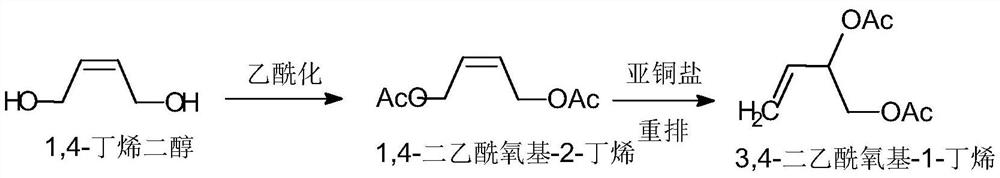
[0035] Esterification step: In the presence of acid, 1,4-butenediol is esterified with acetic acid to obtain a solution containing 1,4-diacetoxy-2-butene and acetic acid, and the acetic acid is removed , to obtain 1,4-diacetoxy-2-butene;
[0036] Isomerization step: adding a cuprous catalyst to the 1,4-diacetoxy-2-butene obtained in the esterification step, heating for isomerization rearrangement reaction, and obtaining 3,4-diacetoxy Base-1-butene mixture;
[0037] Purification step: Purify the mixed liquid obtained in the isomerization step to obtain 3,4-diacetoxy-1-butene.
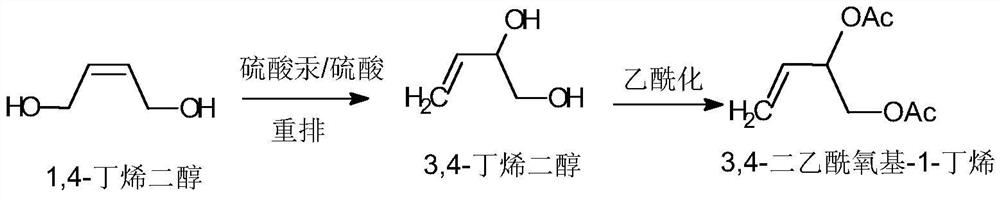
[0038] The specific reaction formula is as follows:
[0039]
[0040] As a preferred embodiment, the specific operating steps of the esterification step are as follows:
[0041] Esterification step 1) adding acetic acid and sulfuric acid, or adding acetic acid and p-toluenesulfonic acid to the reaction ...
Embodiment 1
[0051] A kind of preparation method of 3,4-diacetoxy-1-butene, comprises the following steps:
[0052] Esterification step: add acetic acid and p-toluenesulfonic acid to the reaction vessel; stir to disperse, then add 1,4-butenediol dropwise, and stir for esterification; the molar ratio of acetic acid to 1,4-butenediol is 5:1;. The conditions for the reaction of acetic acid and 1,4-butenediol are as follows: the reaction temperature is 20° C., and the reaction time is 1.0 h. When the reaction generates water, remove the generated water so that the reaction proceeds in the positive direction; after the reaction is completed, remove the acetic acid in the reaction solution to obtain 1,4-diacetoxy-2-butene; specifically, remove The method of the water generated by the reaction is the vacuum distillation water separation method.
[0053]Isomerization step: adding a cuprous catalyst to the 1,4-diacetoxy-2-butene obtained in the esterification step, heating for isomerization rearr...
Embodiment 2
[0057] A kind of preparation method of 3,4-diacetoxy-1-butene, comprises the following steps:
[0058] Esterification step: add acetic acid and sulfuric acid, or add acetic acid and p-toluenesulfonic acid to the reaction vessel; stir and disperse, then add 1,4-butenediol dropwise, and stir for esterification reaction; acetic acid and 1,4-butene The molar ratio of enediol is 2:1; the conditions for the reaction of acetic acid and 1,4-butenediol are: the reaction temperature is 110°C, and the reaction time is 5h. When the reaction generates water, remove the generated water so that the reaction proceeds in the positive direction; after the reaction is completed, remove the acetic acid in the reaction solution to obtain 1,4-diacetoxy-2-butene; specifically, remove The method of the water generated by the reaction is the atmospheric pressure distillation method.
[0059] Isomerization step: adding a cuprous catalyst to the 1,4-diacetoxy-2-butene obtained in the esterification ste...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com