Ion liquid cross-linked polymer supported nanometer palladium metal catalytic material, preparation method and applications thereof
A technology of cross-linked polymers and ionic liquids, which is applied in the preparation of amino compounds, organic compounds, and aminohydroxyl compounds. It can solve the problems of unreviewed catalyst reusability, complex catalyst preparation process, and harsh reaction conditions. , to achieve the effect of good reusability, suitable for large-scale production, and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
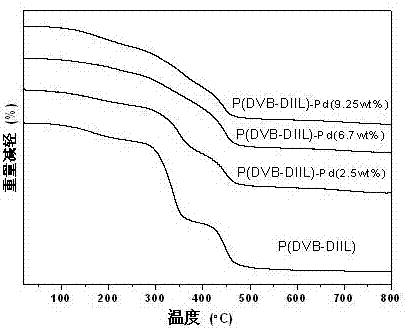
[0044] Preparation of nano-palladium metal catalytic material P(DVB-DIIL)-Pd (2.5 wt%) supported by ionic liquid crosslinked polymer with 2.5% palladium mass content
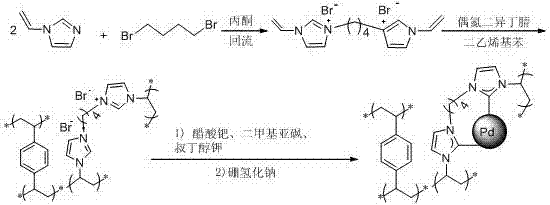
[0045] (1) Synthesis of biimidazole-based ionic liquid bromide 1,4-bis[3-(1-vinylimidazole)]butane
[0046] Under argon atmosphere, in a 100 mL round bottom flask, add 1-vinylimidazole (9.412 g, 0.10 mol), 1,4-dibromobutane (10.80 g, 0.05 mol) and 50 mL of acetone, at 60 Stir at ˚C for 24 h. After the reaction was complete, the white precipitate was washed successively with chloroform (30 mL×3) and acetone (30 mL×3). The product was dried under vacuum at 40 ˚C for 12 h to obtain 1,4-bis[3-(1-vinylimidazole)]butane bromide.
[0047] (2) Preparation of biimidazole-based ionic liquid and cross-linked copolymer carrier material P(DVB-DIIL)
[0048] Under an argon atmosphere, in a 100ml reaction flask, add divinylbenzene (DVB) (1.302 g, 10.0 mmol), 1,4-bis[3-(1-vinylimidazole)]butane bromide (4.041 g , 10.0 mmol)...
Embodiment 2
[0052] Preparation of nano-palladium metal catalytic material P(DVB-DIIL)-Pd (6.7wt%) supported by ionic liquid cross-linked polymer with palladium mass content of 6.7%
[0053] (1) Synthesis of biimidazole-based ionic liquid bromide 1,4-bis[3-(1-vinylimidazole)]butane
[0054] Under argon atmosphere, in a 100 mL round bottom flask, add 1-vinylimidazole (9.412 g, 0.10 mol), 1,4-dibromobutane (10.80 g, 0.05 mol) and 50 mL of acetone, at 60 Stir at ˚C for 24 h. After the reaction was complete, the white precipitate was washed successively with chloroform (30 mL×3) and acetone (30 mL×3). The product was dried under vacuum at 40 ˚C for 12 h to obtain 1,4-bis[3-(1-vinylimidazole)]butane bromide.
[0055] (2) Preparation of biimidazole-based ionic liquid and cross-linked copolymer carrier material P(DVB-DIIL)
[0056] Under an argon atmosphere, in a 100ml reaction flask, add divinylbenzene (DVB) (1.302 g, 10.0 mmol), 1,4-bis[3-(1-vinylimidazole)]butane bromide (4.041 g , 10.0 mm...
Embodiment 3
[0060] Preparation of nano-palladium metal catalytic material P(DVB-DIIL)-Pd (9.25%) supported by ionic liquid cross-linked polymer with palladium mass content of 9.25%
[0061] (1) Synthesis of biimidazole-based ionic liquid bromide 1,4-bis[3-(1-vinylimidazole)]butane
[0062] Under argon atmosphere, in a 100 mL round bottom flask, add 1-vinylimidazole (9.412 g, 0.10 mol), 1,4-dibromobutane (10.80 g, 0.05 mol) and 50 mL of acetone, at 60 Stir at ˚C for 24 h. After the reaction was complete, the white precipitate was washed successively with chloroform (30 mL×3) and acetone (30 mL×3). The product was dried under vacuum at 40 ˚C for 12 h to obtain 1,4-bis[3-(1-vinylimidazole)]butane bromide.
[0063] (2) Preparation of biimidazole-based ionic liquid and cross-linked copolymer carrier material P(DVB-DIIL)
[0064] Under an argon atmosphere, in a 100ml reaction flask, add divinylbenzene (DVB) (1.302 g, 10.0 mmol), 1,4-bis[3-(1-vinylimidazole)]butane bromide (4.041 g , 10.0 mm...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com