Whole genome sequencing method of influenza B virus
A technology for influenza virus and whole genome amplification, applied in the field of influenza virus RT-PCR whole genome sequencing, can solve the problems of inconvenient operation, long cycle, and high cost of testing the genome of pathogenic microorganisms
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] Example 1: Design and synthesis of type B whole genome sequencing primers
[0041] Download GenBank database (http: / / www.ncbi.nlm.nih.gov / genomes / FLU / FLU.html) from 2013 to 2017 in China and GISAID (http: / / platform.gisaid.org / epi3 / frontend) The HA, NA, PA, PB1, PB2, NP, M, and NS genes of influenza B virus in the database, the software compares and analyzes the consistency of each gene sequence of different viruses, and selects a relatively conserved region to design a genome-wide specific amplification primer sequence . In primer design, 4 or less degenerate bases are allowed at the same variable site. Screen the extracted candidate primers that meet the following requirements: ①The specific amplification sequence length is between 18bp and 26bp; ②Tm value is between 50°C and 62°C; ③GC% is between 40% and 60%; ④polyN ≤4bp; ⑤Hairpin≤4bp; ⑥coverage >90%; ⑦for BLAST screening, specificity score>L×0.4.
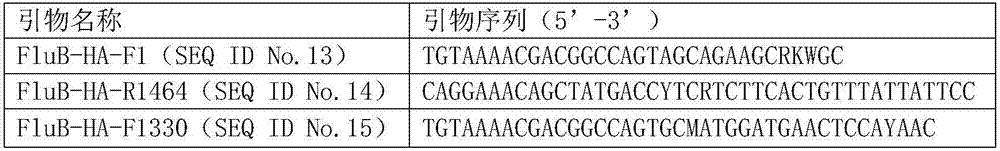
[0042] The primer sequences shown in Table 1 below were obtained...
Embodiment 2
[0046] Embodiment 2: detect unknown virus
[0047] 1. Extraction of viral RNA:
[0048] Take 140 μL sample (plasma, serum, urine, cell culture medium, cell-free body fluid), add it to 560 μL AVL lysate containing 5.6 μg Carrier RNA, follow the instructions of QIAamp Viral RNA Mini Handbook (Qiagen, catalog #52904 / 52906) Viral RNA was extracted with an elution volume of 50 μL.
[0049] 2. rRT-PCR reaction
[0050] 1) System configuration: use Takara's PrimeScript TM One Step RT-PCR Kit Ver.2 (RR055A) reaction solution, the configuration components are shown in Table 2 below:
[0051] Table 2
[0052]
[0053] 2) rRT-PCR: Put the reaction tube with the above reaction system in the PCR machine for rRT-PCR. The reaction procedure is shown in Table 3 below:
[0054] table 3
[0055]
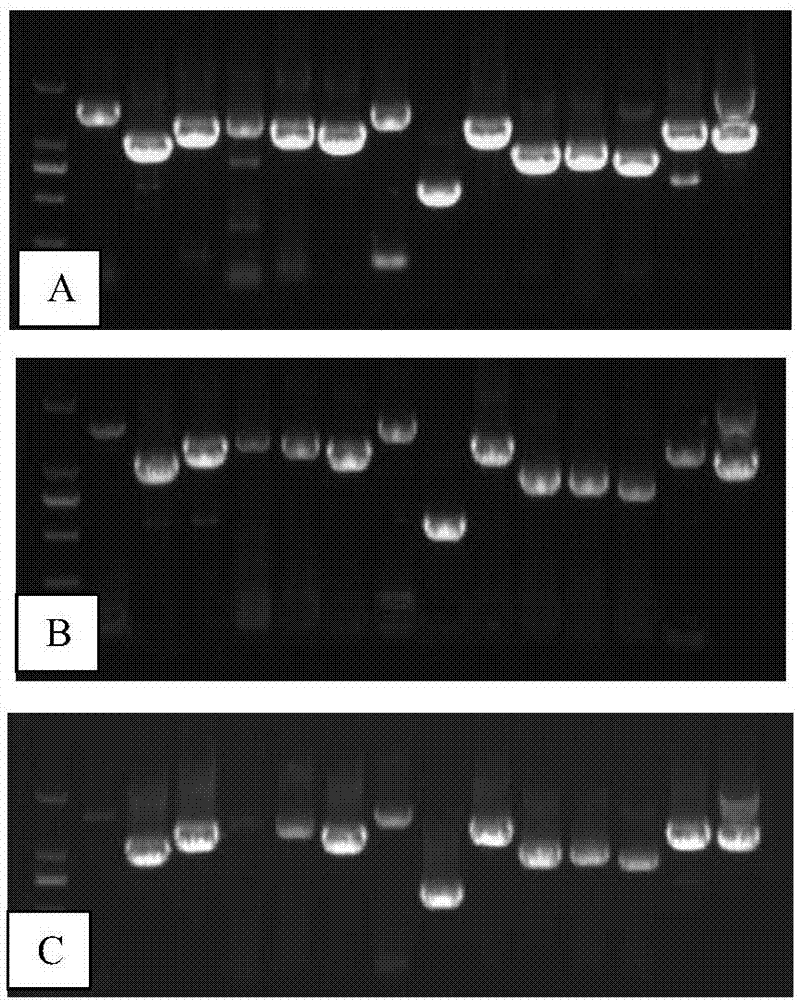
[0056] 3. Detection of RT-PCR products: Take 1 ul of each product in 1% Agrorose gel for electrophoresis to observe the results.
[0057] 4. Judgment of results: When all 14 reaction pr...
Embodiment 3
[0061] Embodiment 3: reclaim RT-PCR product
[0062] The amplified product obtained by the amplification method of Example 2 was recovered by cutting with agarose gel, specifically as follows:
[0063] 1) 25 μl of RT-PCR product was subjected to electrophoresis in 1% agarose gel.
[0064] 2) Cut the agarose gel containing the target fragment, remove the excess agarose gel as much as possible, put the gel containing the target band into a 1.5ml centrifuge tube, and write the number.
[0065] 3) Add 300 ul to 400 ul of QC solution (Qiagen kit, catalog#28706), put in a water bath at 50° C., and invert once every 5 minutes until the sol is completely dissolved.
[0066] 4) Transfer the liquid dissolved in step 3 into a column covered with a collection tube, and centrifuge at 12000 rpm for 1 min.
[0067] 5) Discard the waste liquid, add 500ul of QG solution, and centrifuge at 12000rpm for 1min.
[0068] 6) Discard the waste liquid, add 700ul PE solution (absolute ethanol was ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| cover factor | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com