Mesoporous chromium-based fluorination catalyst and preparation method of same
A base fluorination catalyst and a fluorination catalyst technology are applied in the field of catalyzing the preparation of a chromium base fluorination catalyst for R134a and the field of preparation thereof, which can solve the problem of uneven distribution of catalyst active metals, high selectivity of by-product R134, and uneven distribution of catalyst pore structure. Equalization problem, to achieve the effect of increasing specific surface area, reducing selectivity, and good pore structure
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
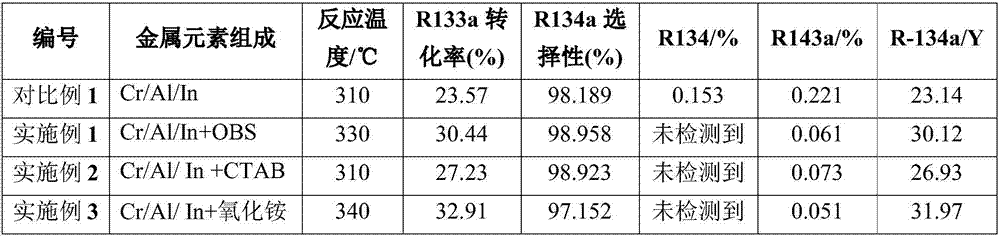
[0048] Weigh 21.78g CrCl respectively 3 ·6H 2 O, 12.84g AlCl 3 , 3.86g InCl 3 1. Dissolve 0.626g OBS in 100mL deionized water, and adjust the pH value to 9 by adding ammonia water. The generated slurry material was transferred to a 200mL stainless steel synthesis kettle and heated to 120°C to continue the reaction for 72h. After the reaction was completed, it was cooled to room temperature. The material was unloaded and washed several times with a centrifuge, and the filter cake was dried at room temperature. Then, it was calcined in a tube furnace at 400°C for 3 hours in a nitrogen atmosphere. The calcined samples were pulverized and sieved, mixed with graphite, and pressed into tablets to obtain a catalytic precursor.
[0049] Then, the catalyst precursor was loaded into a reactor, and a mixed gas of nitrogen and HF was introduced to fluorinate at 340° C. to prepare a chromium-based fluorination catalyst.
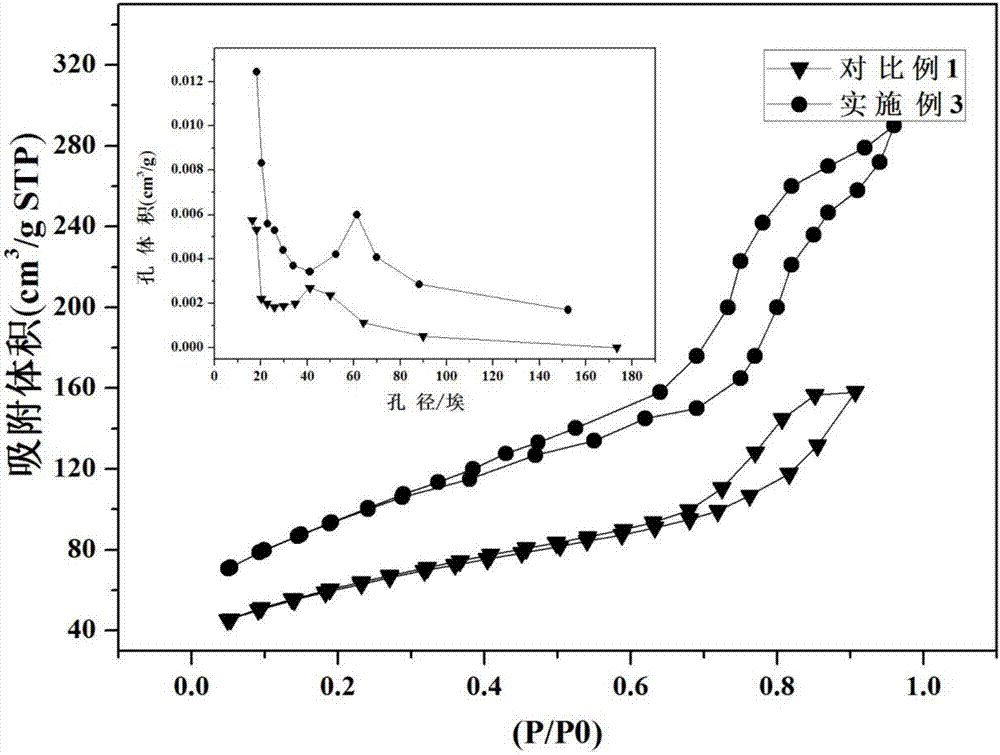
[0050] Using the specific surface area and pore structure tester...
Embodiment 2
[0052] Weigh 34.84g CrCl respectively 3 ·6H 2 O, 10.86g AlCl 3 , 3.86g InCl 3 1. Dissolve 0.364g of trimethylhexadecylammonium bromide (CTAB) in 90mL of deionized water, and adjust the pH value to 8 by adding ammonia water. The generated slurry material was transferred to a 200mL stainless steel synthesis kettle and heated to 110°C to continue the reaction for 80h. After the reaction was completed, it was cooled to room temperature. The material was unloaded and washed several times with a centrifuge, and the filter cake was dried at room temperature. Then, it was calcined at 350° C. for 4 h in a tube furnace under a nitrogen atmosphere. The calcined samples were pulverized and sieved, mixed with graphite, and pressed into tablets to obtain a catalytic precursor.
[0053] Then, the catalyst precursor was loaded into a reactor, and a mixed gas of nitrogen and HF was introduced to fluorinate at 330° C. to prepare a chromium-based fluorination catalyst.
[0054] Using the spe...
Embodiment 3
[0056] Weigh 17.42g CrCl respectively 3 ·6H 2 O, 14.82g AlCl 3 , 3.86g InCl 3 1. Dissolve 0.948g of ammonium oxide in 80mL of deionized water, and adjust the pH value to 10 by adding ammonia water. The generated slurry material was transferred to a 200mL stainless steel synthesis kettle and heated to 130°C to continue the reaction for 80h. After the reaction was completed, it was cooled to room temperature, and the material was unloaded and washed several times with a centrifuge, and the filter cake was dried at room temperature. Then, it was calcined in a tube furnace at 400°C for 3 hours in a nitrogen atmosphere. The calcined samples were pulverized and sieved, mixed with graphite, and pressed into tablets to obtain a catalytic precursor.
[0057] Then, the catalyst precursor was loaded into a reactor, and a mixed gas of nitrogen and HF was introduced to fluorinate at 320° C. to prepare a chromium-based fluorination catalyst.
[0058] Using the specific surface area and ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| pore size | aaaaa | aaaaa |
| specific surface area | aaaaa | aaaaa |
| pore size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com