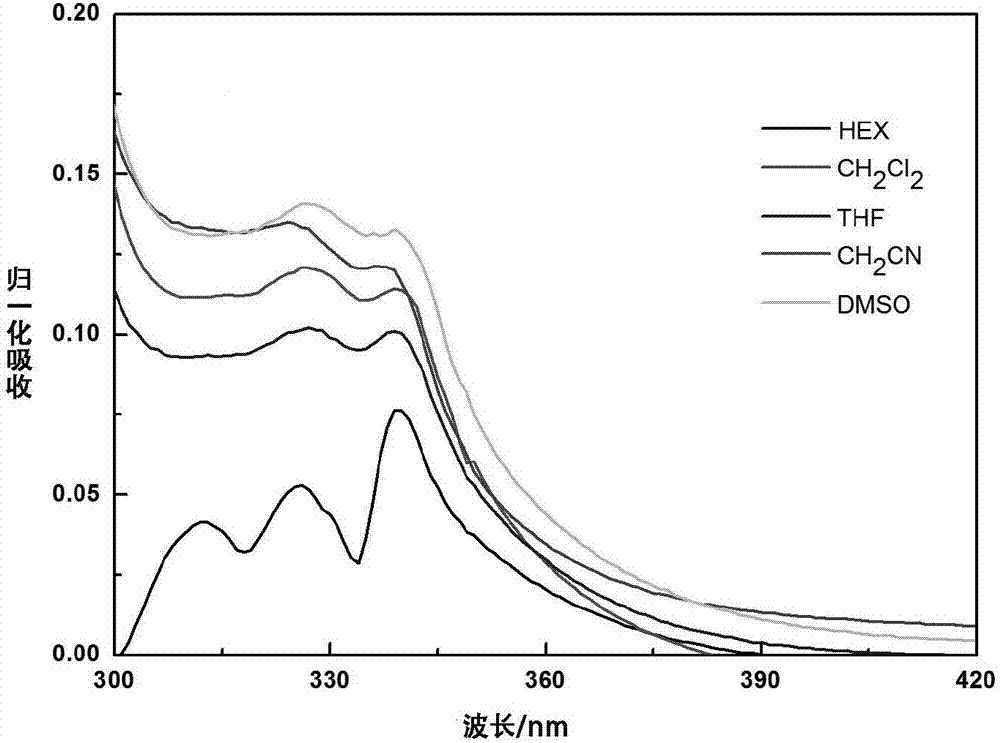
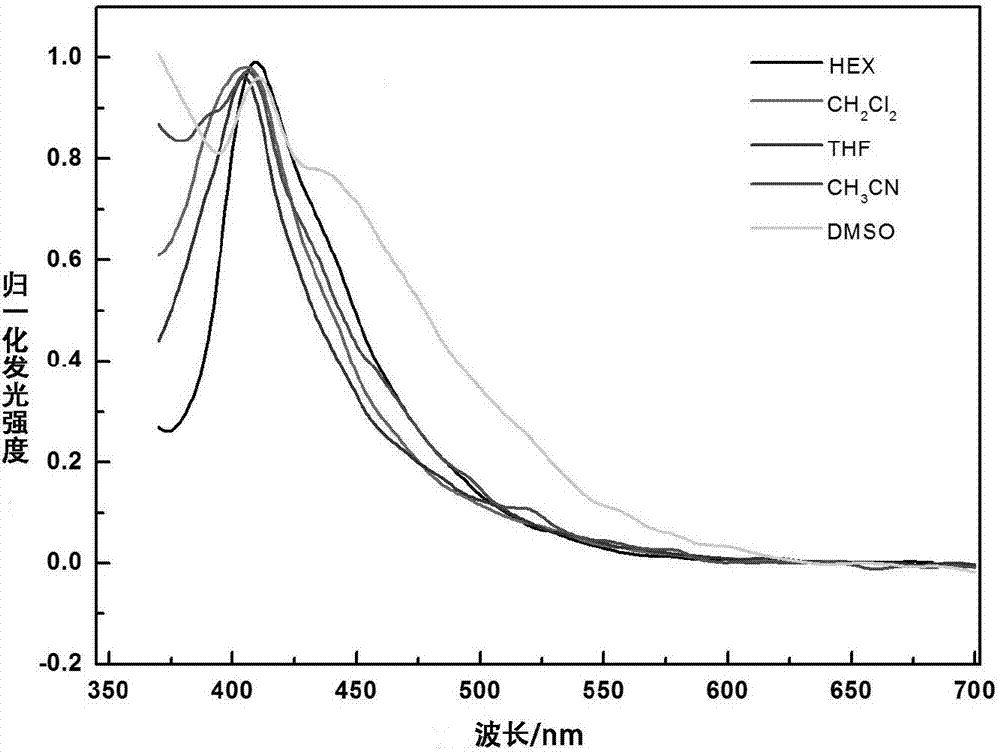
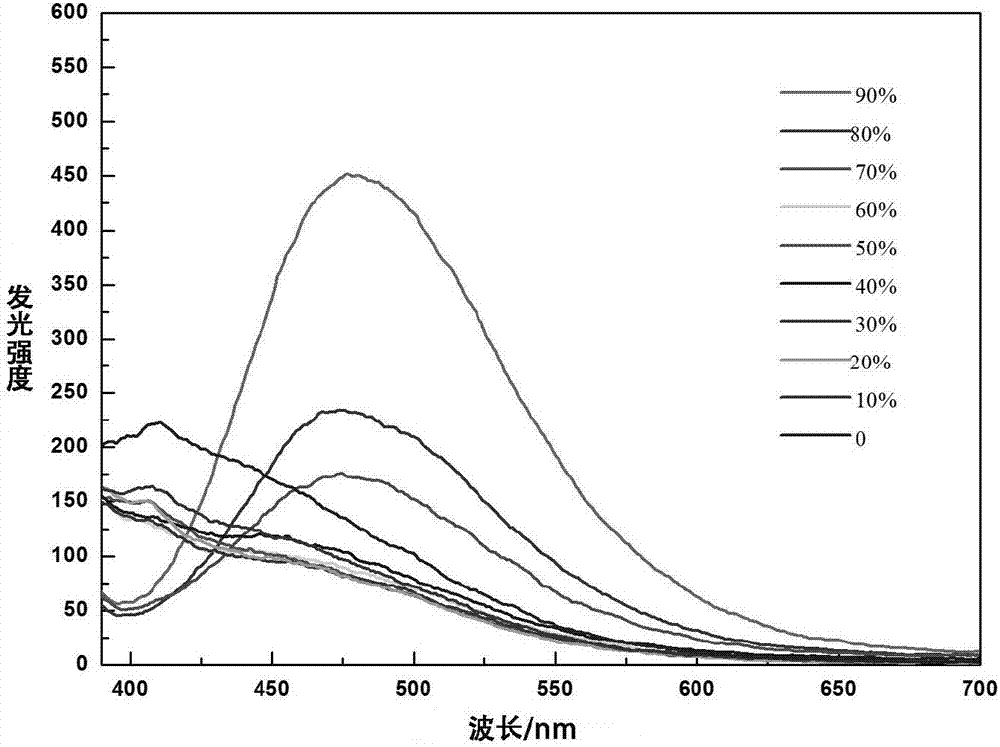
Dicarbazole compound containing tetraphenylethylene structure as well as preparation and application thereof
A technology of tetraphenylethylene and biscarbazole, which is applied in the fields of chemical instruments and methods, organic chemistry, organic semiconductor device materials, etc., can solve the problems of weak luminescence and no luminescence, and achieve easy operation, high luminous efficiency, The effect of broad application prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Example 1. The preparation of 9,9'-(5-(2,2-diphenyl-1-(4-phenylmethyl))vinyl-1,3-phenylene)biscarbazole, the steps are as follows :
[0029] (1) Synthesis of 3,5-bis(9-carbazolyl)phenyl-(4-methylphenyl)methanone;
[0030] At -78°C, 18.8 mL of tert-butyllithium (1.3 M , 24.4mmol), stirred at this temperature for 1h, then stirred at room temperature for 5h; then the temperature of the mixture was lowered to -78°C, and 3.9mL (32.7mmol) of p-tolualdehyde was added to the mixture; the reactant Gradually return to room temperature, stir the reaction overnight at room temperature; quench the reaction with ice water, stop stirring, CH 2 Cl 2 Extract 3 times, wash 3 times with water, combine the organic layers, wash with anhydrous MgSO 4 Drying and spin-drying the solvent; and then separated by column chromatography to obtain 1.9 g of compound 3,5-bis(9-carbazolyl)phenyl-(4-methylphenyl)methanone as a white solid, with a yield of 36.5%;
[0031] (2) Synthesis and characteri...
Embodiment 2
[0034] Embodiment 2. Structure, preparation and performance of organic electroluminescent devices:
[0035] The device we prepared is a blue-green organic electroluminescent device, and the device structure is: ITO / HATCN (thickness 20nm) / NPB (thickness 40nm) / compound DPPBC (thickness 20nm) / TPBI (thickness 40nm) / LiF (thickness 1nm) / Al (thickness 100nm). Among them, HATCN is used as a hole injection layer, and NPB is used as a hole transport layer; 9,9'-(5-(2,2-diphenyl-1-(4-benzyl))vinyl-1,3- Phenylene) biscarbazole (compound DPPBC) as the light emitting layer; TPBI as the electron transport layer.
[0036] Preparation steps of the device: first scrub the ITO glass with acetone, then rinse it with clean water, then ultrasonically clean it with cleaning solution, and finally clean it ultrasonically with clean water. The cleaned substrates were blown dry with nitrogen, and finally treated with UV-ozone to fully clean the surface. According to the structure of the designed dev...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com