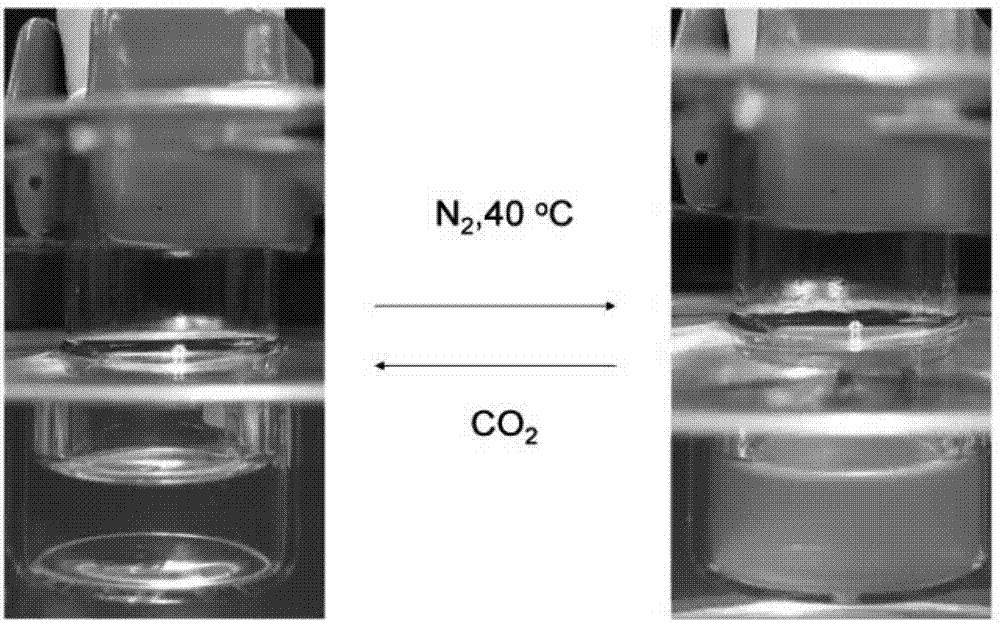
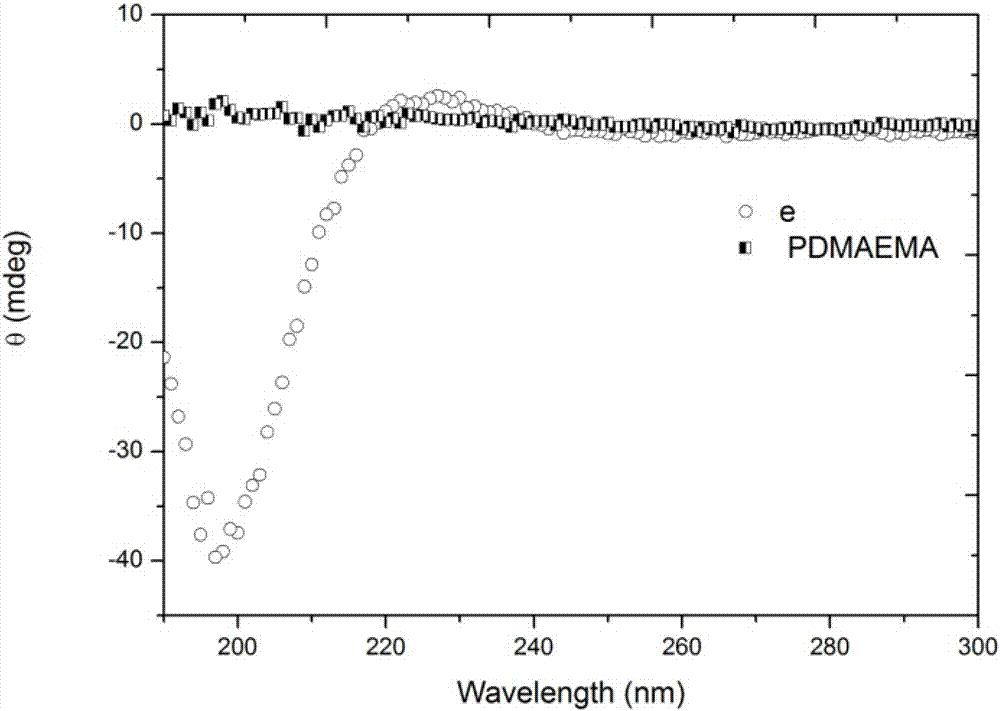
Chiral CO2 responsive vinyl amino acid polymer and preparation method thereof
A polymer and vinyl technology, applied in the field of chiral CO2-responsive vinyl amino acid polymers and their preparation, can solve the problems of poor polymer stability, many steps, poor biocompatibility, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0076] Weigh 0.91 g (equivalent to 0.01 mol) of acryloyl chloride and 2.78 g (equivalent to 0.01 mol) of glycine-L-leucine benzyl ester in tetrahydrofuran to obtain 3.3 g (equivalent to 0.01 mol) N - Acryloyl-glycine-L-leucine benzyl ester. 3.3 g (equivalent to 0.01 mol) N -Acryloyl-glycine-L-leucine benzyl ester and 1.76 g (equivalent to 0.02mol) N,N-dimethylethylenediamine were added to 10 ml N,N-dimethylformamide, and N 2 Deoxygenation. Add 1.0 g catalyst 2-picoline, N 2 Reaction at 30°C for 72h under protection. After the reaction was completed, the solvent and by-products were distilled off under reduced pressure, and the solvent recrystallization with a volume ratio of tetrahydrofuran:n-hexane=1:8 obtained 1.4 g monomer N -Acryloyl-Glycine-L-Leucine- N,N - Dimethylaminoacetamide (monomer c, A-1). 1 H NMR (CDCl 3 ): σ = 0.91 (d, 6H, -CH(C H 3 ) 2 ), 1.51 (m,1H,-C H (CH 3 ) 2 ),1.63 (m, 2H,-C H 2 CH-), 2.22 (m,6H, -N(C H 3 ) 2 ), 2.40 (t, 2H, -C H 2 N(C...
Embodiment 2
[0080] In this example, except monomer c is N -Acryloyl-Glycine-L-Leucine- N,N -Diethylaminoacetamide (monomer c, A-2), the resulting polymer e is poly( N -Acryloyl-Glycine-L-Leucine- N,N -diethylaminoacetamide), the preparation method of monomer c is as follows, and other implementation methods are all the same as in Example 1.
[0081] The preparation method of monomer c in this example is:
[0082] Weigh 0.91 g (equivalent to 0.01 mol) of acryloyl chloride and 2.78 g (equivalent to 0.01 mol) of glycine-L-leucine benzyl ester in dichloromethane to obtain 3.3 g (equivalent to 0.01 mol) N - Acryloyl-glycine-L-leucine benzyl ester. 3.3 g (equivalent to 0.01 mol) N -Acryloyl-glycine-L-leucine benzyl ester and 4.65 g (equivalent to 0.04mol ) N,N-diethylethylenediamine were added to 8 ml N,N-dimethylacetamide, and passed N 2 Deoxygenation. Add 1.0 g catalyst 4-dimethylaminopyridine, N 2 Under the protection of 40 ℃ reaction 48h. After the reaction was completed, the solv...
Embodiment 3
[0084] In this example, except monomer c is N -Acryloyl-Glycine-L-Leucine- N,N -Dimethylaminopropionamide (monomer c, A-3), the obtained polymer e is poly(N-acryloyl-glycine-L-leucine-N, N-dimethylaminopropionamide), monomer The preparation method of c is as follows, and other implementation methods are all the same as in Example 1.
[0085] The preparation method of monomer c in this example is:
[0086] Weigh 0.91 g (equivalent to 0.01 mol) of acryloyl chloride and 2.78 g (equivalent to 0.01 mol) of glycine-L-leucine benzyl ester in dioxane to obtain 3.3 g (equivalent to 0.01 mol) N - Acryloyl-glycine-L-leucine benzyl ester. 3.3 g (equivalent to 0.01 mol) N -Acryloyl-glycine-L-leucine benzyl ester and 8.16 g (equivalent to 0.08 mol ) 3-(dimethylamino)propylamine were added to 3 ml of N-methylpyrrolidone, passed through N 2 Deoxygenation. Add 1.5 g catalyst 2-hydroxypyridine, N 2 Reacted at 45°C for 40 h under protection. After the reaction was completed, the solvent ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| degree of polymerization | aaaaa | aaaaa |
| degree of polymerization | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com