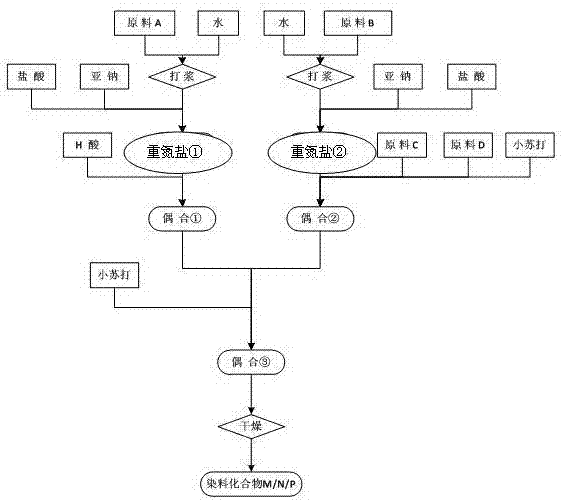
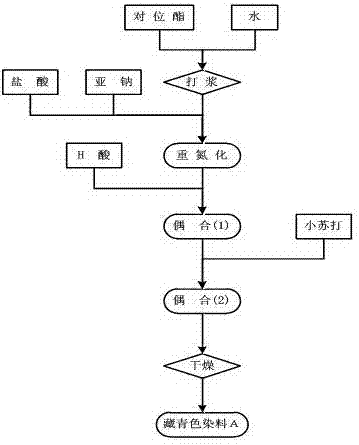
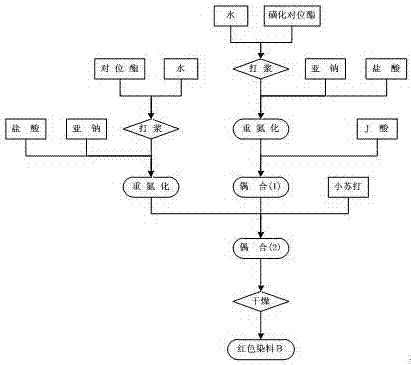
Synthesis method of black active dyestuff compound
A technology for reactive dyes and a synthesis method, which is applied in the synthesis field of black reactive dye compounds, can solve the problems of difficult removal of by-products, high preparation cost, low yield and the like, and achieves the elimination of processes and equipment, reduction of reaction equipment occupancy rate, less time effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0066] 1. Diazonium salt①
[0067] With the p-(β-sulfate ethyl sulfone) aniline of 2.51mol parts (being commonly called as p-position ester, R in formula (1) 1 =-H,Z 1 =-SO 2 CH 2 CH 2 OSO 3 H) Make a slurry in an appropriate amount of water, add hydrochloric acid and stir evenly, then cool down to 0-5°C, then add 30% sodium nitrite solution for diazotization reaction, keep T=3-10°C throughout the diazotization process , and keep both hydrochloric acid and sodium nitrite in excess. After 1 hour of heat preservation reaction, use sulfamic acid to balance the excess sodium nitrite, diazonium salt ① for later use, reaction time: 2.5 hours;
[0068] 2. Diazonium salt②
[0069] The 4-(beta-hydroxyethylsulfone-sulfate) aniline-2-sulfonic acid of 0.425mol part (being commonly called as sulfonated p-position ester, R in formula (2) 2 =-SO 3 H, Z 2 =-CH 2 CH 2 OSO 3 H) Make a slurry in an appropriate amount of water, add hydrochloric acid and stir evenly, then cool down t...
Embodiment 2
[0079] 1. Diazonium salt①
[0080] The p-(beta-sulfate ethyl sulfone) aniline (being commonly called as p-position ester of 2.63mol parts, R in formula (1) 1 =-H,Z 1 =-SO 2 CH 2 CH 2 OSO 3 H) Make a slurry in an appropriate amount of water, add hydrochloric acid and stir evenly, then cool down to 0-5°C, then add 30% sodium nitrite solution for diazotization reaction, keep T=3-10°C throughout the diazotization process , and keep both hydrochloric acid and sodium nitrite in excess, after heat preservation reaction for 1 hour, balance the excess sodium nitrite with sulfamic acid, diazonium salt ① Standby, reaction time: 2.5 hours;
[0081] 2. Diazonium salt②
[0082] With the 4-(β-hydroxyethylsulfone-sulfate) aniline-2-sulfonic acid of 0.43mol part (being commonly called as sulfonated p-position ester, R in formula (2) 2 =-SO 3 H, Z 2 =-CH 2 CH 2 OSO 3 H) and 0.17 parts of anthranilic acid (R in formula (2) 2 =-SO 3 H, without Z 2 substituent) in an appropriate am...
Embodiment 3
[0091] 1. Diazonium salt①
[0092] The p-(beta-sulfate ethyl sulfone) aniline of 2.65mol parts (being commonly called as p-position ester, R in formula 1 1 =-H,Z 1 =-SO 2 CH 2 CH 2 OSO 3 H,) in an appropriate amount of water to beat evenly, add hydrochloric acid and stir evenly, then cool down to 0~5°C, then add 30% sodium nitrite solution to carry out diazotization reaction, and keep T=3~10 throughout the diazotization process ℃, and keep both hydrochloric acid and sodium nitrite in excess. After 1 hour of heat preservation reaction, use sulfamic acid to balance the excess sodium nitrite, diazonium salt ① for later use, reaction time: 2.5 hours;
[0093] 2. Diazonium salt②
[0094]With 0.05mol part of 4-(β-hydroxyethylsulfone-sulfate) aniline-2-sulfonic acid (commonly known as sulfonated para-ester, R in formula 2 2 =-SO 3 H, Z 2 =-CH 2 CH 2 OSO 3 H,) and 0.26mol parts of anthranilic acid (R in formula (2) 2 =-SO 3 H, no Z 2 substituent) in an appropriate amo...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com