A kind of porous heavy metal ion adsorbent and preparation method thereof
A technology of heavy metal ions and adsorbents, applied in the directions of alkali metal compounds, chemical instruments and methods, adsorbed water/sewage treatment, etc., can solve the problems of difficult to uniformly disperse polymer materials, poor heavy metal adsorption effect, and low elution efficiency. , to achieve the effect of great potential for industrial application, easy desorption and regeneration, and rapid adsorption
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
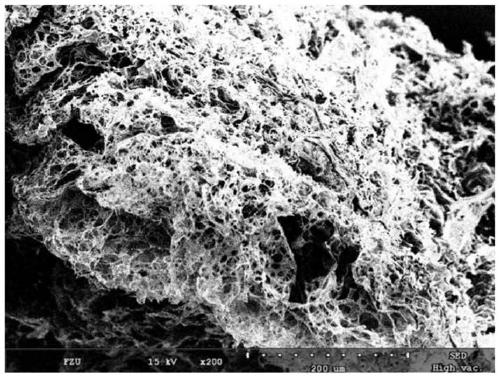
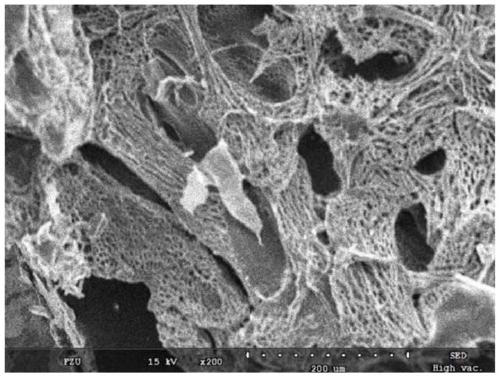
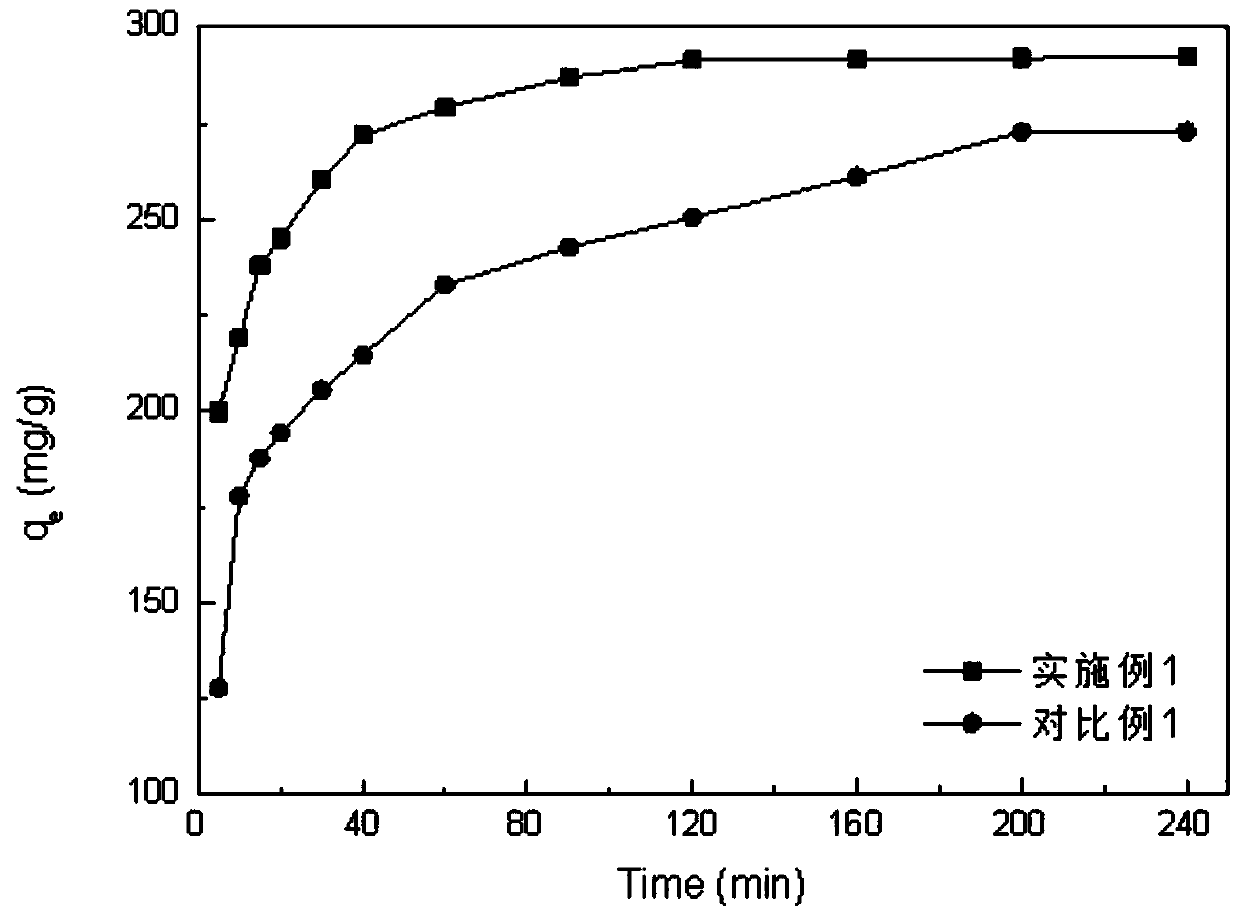
Image
Examples
Embodiment 1
[0034] A kind of preparation method of porous heavy metal ion adsorbent, it comprises the steps:
[0035] (1) Add 2 parts of glacial acetic acid and 24 parts of absolute ethanol to 10 parts of butyl titanate in sequence, and stir and mix evenly to obtain solution A;
[0036] (2) Add 1 part of deionized water to 7.8 parts of absolute ethanol, add two drops of concentrated hydrochloric acid dropwise, and mix uniformly to obtain solution B;
[0037] (3) Slowly stir the solution A prepared in step (1) and pour it into the solution B prepared in step (2), and continue stirring at room temperature for 15 minutes, then carry out standing treatment for 2 hours to obtain a titanium dioxide sol;
[0038] (4) Add 1.95 parts of sodium hydroxide to 7.5 parts of deionized water and stir and mix evenly to obtain a sodium hydroxide solution, then stir and drop 5 parts of acrylic acid into the prepared sodium hydroxide solution under ice-water bath conditions , then add 0.02 parts of N,N'-met...
Embodiment 2
[0051] A kind of preparation method of porous heavy metal ion adsorbent, it comprises the steps:
[0052] (1) Add 2 parts of glacial acetic acid and 24 parts of absolute ethanol to 10 parts of butyl titanate in sequence, and stir and mix evenly to obtain solution A;
[0053] (2) Add 0.1 part of deionized water to 7.8 parts of absolute ethanol, add two drops of concentrated hydrochloric acid dropwise, and mix uniformly to obtain solution B;
[0054] (3) Slowly stir the solution A prepared in step (1) and pour it into the solution B prepared in step (2), and continue stirring at room temperature for 15 minutes, then carry out standing treatment for 2 hours to obtain a titanium dioxide sol;
[0055] (4) Add 1.56 parts of sodium hydroxide to 7.5 parts of deionized water and stir and mix evenly to obtain a sodium hydroxide solution, then stir and drop 4 parts of acrylic acid into the prepared sodium hydroxide solution under ice-water bath conditions , then add 0.004 parts of N,N'-...
Embodiment 3
[0060] A kind of preparation method of porous heavy metal ion adsorbent, it comprises the steps:
[0061] (1) Add 5 parts of glacial acetic acid and 24 parts of absolute ethanol to 10 parts of butyl titanate in sequence, and stir and mix evenly to obtain solution A;
[0062] (2) Add 0.9 parts of deionized water to 7.8 parts of absolute ethanol, add two drops of concentrated hydrochloric acid dropwise, and stir and mix evenly to obtain solution B;
[0063] (3) Slowly stir the solution A prepared in step (1) and pour it into the solution B prepared in step (2), and continue stirring at room temperature for 15 minutes, then carry out standing treatment for 2 hours to obtain a titanium dioxide sol;
[0064] (4) Add 2.34 parts of sodium hydroxide to 10 parts of deionized water and stir and mix uniformly to obtain a sodium hydroxide solution, then stir and drop 6 parts of acrylic acid into the prepared sodium hydroxide solution under ice-water bath conditions , then add 0.08 parts ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| adsorption capacity | aaaaa | aaaaa |
| adsorption capacity | aaaaa | aaaaa |
| adsorption capacity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com