Method for preparing nickel-tungsten bimetallic carbide catalyst by organic-inorganic hybrid route and application of nickel-tungsten bimetallic carbide catalyst
A bimetallic carbide and catalyst technology, which is applied in the direction of catalyst activation/preparation, hydrocarbon production from oxygen-containing organic compounds, physical/chemical process catalysts, etc., can solve the problem of reducing catalyst active sites, limiting carbide materials, and polymer carbon Pollution and other issues, to achieve the effect of optimistic industrial application prospects, simple operation, and energy-saving product particles
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
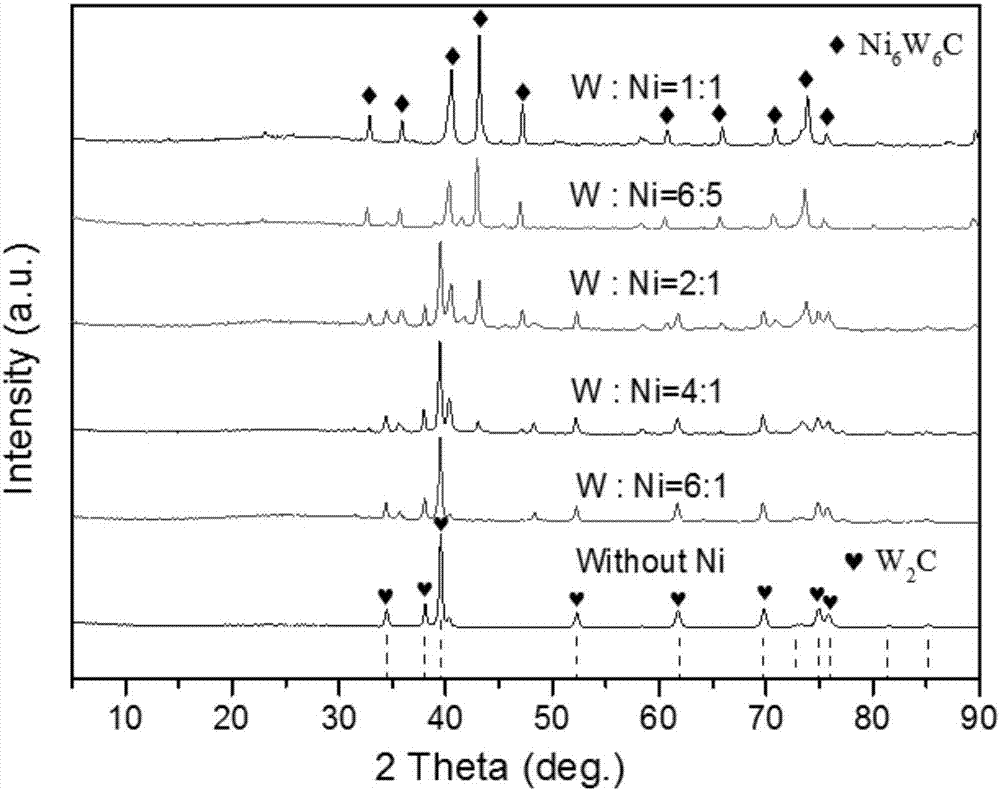
[0024] 0.43g melamine (C 3 N 3 (NH 2 ) 3 ) and 1g ammonium metatungstate ((NH 4 ) 6 h 2 W 12 o 40 ·nH 2 O) Add 150 mL of deionized water, and heat to 70° C. under magnetic stirring. After the melamine and ammonium metatungstate are completely dissolved, raise the temperature to 90°C, slowly form a white milky liquid, keep stirring at 90°C for 12 hours, then collect the white precipitate from the suspension by centrifugation, wash with deionized water and ethanol After several times, dry in an oven at 80°C for 24 hours to obtain ammonium metatungstate-melamine hybrid.
[0025] 0.75g Ni(NO 3 ) 2 ·6H 2 O was dissolved in 1.6 mL of water, then added dropwise to a tube containing 1 g of the hybrid, sonicated for 30 min, and then dried at 90 °C for 24 h. An organic-inorganic hybrid having an atomic ratio of W:Ni=1 is thus obtained.
[0026] The ammonium metatungstate-melamine hybrid (1 g) containing nickel nitrate was placed in a quartz boat in a quartz tube reactor an...
Embodiment 2
[0028] 4.3g melamine (C 3 N 3 (NH 2 ) 3 ) and 20g sodium metatungstate ((NH 4 ) 6 h 2 W 12 o 40 ·nH 2 O) Add 3050mL of deionized water and heat to 70°C under magnetic stirring. After the melamine and ammonium metatungstate are completely dissolved, raise the temperature to 80°C, slowly form a white milky liquid, keep stirring at 80°C for 24 hours, then collect the white precipitate from the suspension by centrifugation, wash with deionized water and ethanol After several times, dry in an oven at 80°C for 24 hours to obtain ammonium metatungstate-melamine hybrid.
[0029] 0.375g Ni(NO 3 ) 2 ·6H 2 O was dissolved in 1.6 mL of water, then added dropwise to a tube containing 1 g of the hybrid, sonicated for 50 min, and then dried at 90 °C for 24 h. An organic-inorganic hybrid having an atomic ratio of W:Ni=2 is thus obtained.
[0030] The ammonium metatungstate-melamine hybrid (1 g) containing nickel nitrate was placed in a quartz boat in a quartz tube reactor and py...
Embodiment 3
[0032] 1g melamine (C 3 N 3 (NH 2 ) 3 ) and 0.2g ammonium metatungstate ((NH 4 ) 6 h 2 W 12 o 40 ·nH 2 O) Add 150mL of deionized water and heat to 60°C under magnetic stirring. After the melamine and ammonium metatungstate are completely dissolved, raise the temperature to 95°C, slowly form a white milky liquid, keep stirring at 95°C for 6 hours, then collect the white precipitate from the suspension by centrifugation, wash with deionized water and ethanol After several times, dry in an oven at 80°C for 24 hours to obtain ammonium metatungstate-melamine hybrid.
[0033] 0.1875g Ni(NO 3 ) 2 ·6H 2 O was dissolved in 1.6 mL of water, then added dropwise to a tube containing 1 g of the hybrid, sonicated for 90 min, and then dried at 90 °C for 24 h. An organic-inorganic hybrid having an atomic ratio of W:Ni=4 is thus obtained.
[0034] The ammonium metatungstate-melamine hybrid (1 g) containing nickel nitrate was placed in a quartz boat in a quartz tube reactor and py...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com