A kind of hydrogel for preventing abdominal postoperative adhesion and its preparation method and application
A technology of hydrogel and peritoneal cavity, which is applied in the field of hydrogel and its preparation to prevent postoperative adhesions after abdominal surgery. It can solve the problems of fibrin immunogenicity risk, difficulty in using surgical laparoscopy, and limited effectiveness. Inhibits the invasion of fibroblasts, prevents the occurrence of adhesions, and promotes healing
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
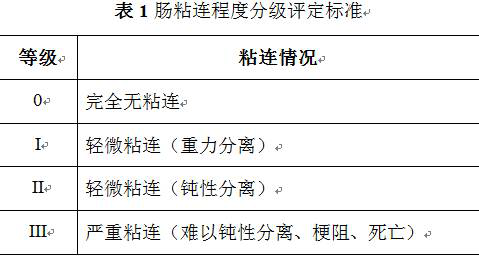
[0028] All animals were fasted without food and water for 12 hours; they were anesthetized by intraperitoneal injection of chloral hydrate. After successful anesthesia, the rats were fixed supine on the operating board, the abdomen was depilated, routinely disinfected, sterile towels were spread, and the central position of the abdomen was removed to create an incision. For the cecum, find and take out the terminal position of the small intestine, select the position above the terminal end of the small intestine, place it on sterile gauze, and stuff it back into the cecum to dry the serosa, gently scrape the surface of the ileum membrane with a scalpel blade, and then drop absolute ethanol on the wound surface , and then grasp the mesenteric artery with toothless forceps, causing temporary ischemia. In the control group, anti-adhesion materials were not applied to the operation area, and the ileocecal gyrus was brought into the original position of the abdominal cavity, and the...
Embodiment 2
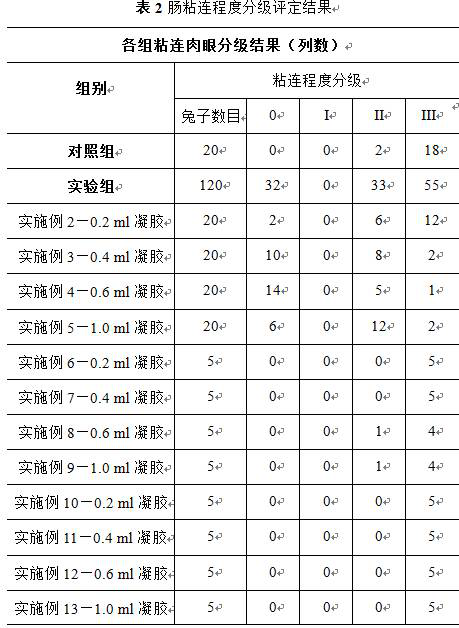
[0031] Take calcium chloride 4 wt%, sodium alginate (1.2 wt%) / sodium carboxymethyl cellulose (2 wt%) as an example (37°C, G' / G'' : 1600 / 500 Pa / Pa, n : 1700 Pa S), weighed 2.4 g sodium alginate and 4 g sodium carboxymethyl cellulose, respectively dissolved in 100 ml deionized water to obtain 100 ml sodium alginate solution and 100 ml sodium carboxymethyl cellulose solution, and 100 ml Sodium alginate solution and 100ml sodium carboxymethylcellulose solution were mixed, and magnetically stirred until uniform to obtain 200ml compound solution c, and 5ml 4wt% calcium chloride solution (cationic aqueous solution d) was added dropwise to compound solution c, made into hydrogels. After standing at room temperature for 1 h, put them in the upper layer of the refrigerator to refrigerate for later use (experimental group 1).
[0032] All animals were fasted without food and water for 12 hours; they were anesthetized by intraperitoneal injection of chloral hydrate. After successful a...
Embodiment 3
[0034] Take calcium chloride 4 wt%, sodium alginate (1.2 wt%) / sodium carboxymethyl cellulose (2 wt%) as an example (37°C, G' / G'' : 1600 / 500 Pa / Pa, n : 1700 Pa S), weighed 2.4 g sodium alginate and 4 g sodium carboxymethyl cellulose, respectively dissolved in 100 ml deionized water to obtain 100 ml sodium alginate solution and 100 ml sodium carboxymethyl cellulose solution, and 100 ml Sodium alginate solution and 100ml sodium carboxymethylcellulose solution were mixed, and magnetically stirred until uniform to obtain 200ml compound solution c, and 5ml 4wt% calcium chloride solution (cationic aqueous solution d) was added dropwise to compound solution c, made into hydrogels. After standing at room temperature for 1 h, put them in the upper layer of the refrigerator to refrigerate for later use (experimental group 1).
[0035] All animals were fasted without food and water for 12 hours; they were anesthetized by intraperitoneal injection of chloral hydrate. After successful...
PUM
| Property | Measurement | Unit |
|---|---|---|
| storage modulus | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com