Composite amido weak-base aion exchange resin and method for recycling rhenium from arsenic sulfide residue extracting solution
A weakly basic anion and exchange resin technology, which is applied in anion exchange, ion exchange, chemical instruments and methods, etc., can solve the problems of complicated rhenium recovery process and low recovery rate, and achieve good product stability, environmental protection, and The effect of reducing the loss of rhenium
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] The composite amine-based weakly basic anion exchange resin of the present embodiment is prepared by a preparation method comprising the following steps:
[0036] Control the reaction temperature at 50°C, mix chloromethylated polystyrene-divinylbenzene cross-linked resin (chlorine balls) with diethanolamine, N,N-dioctylamine, N-methylimidazole, and diglycolic anhydride in Stir and react in methanol for 8 hours, filter after the reaction, wash with methanol three times, and dry the resin in vacuum at 55°C to obtain; The mass ratio of diglycolic anhydride is 10:10:25:10:10.
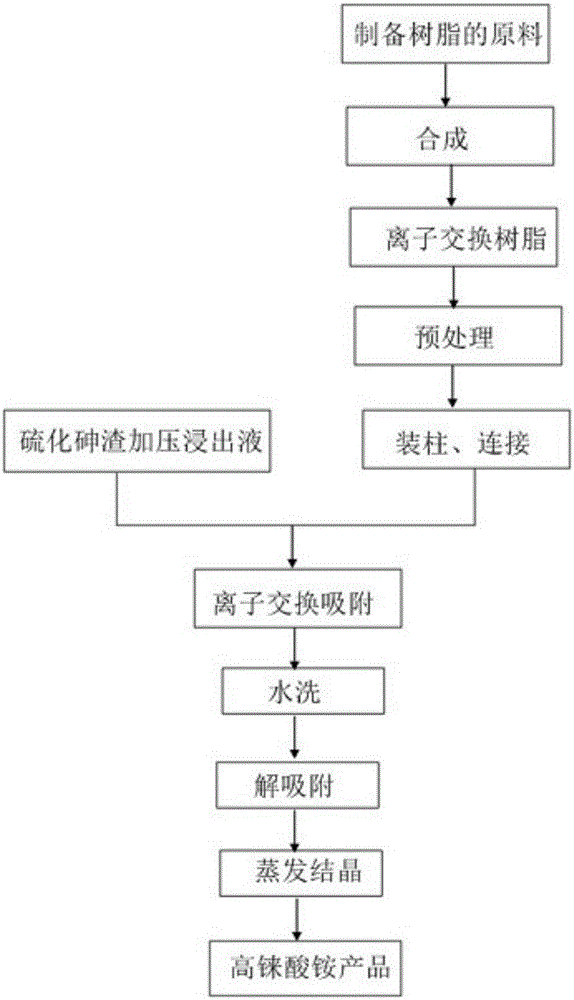
[0037] This embodiment adopts the method of reclaiming rhenium from the leaching solution of arsenic sulfide slag using the above-mentioned composite amine-based weakly basic anion exchange resin, such as figure 1 shown, including the following steps:
[0038] 1) Take 1L of the above composite amine-based weakly basic anion exchange resin and put them into 4 ion exchange columns connected in series...
Embodiment 2
[0042] The composite amine-based weakly basic anion exchange resin of the present embodiment is prepared by a preparation method comprising the following steps:
[0043] Mix chloromethylated polystyrene-divinylbenzene cross-linked resin (chlorine balls) with diethanolamine, N,N-dioctylamine, N-methylimidazole, and diglycolic anhydride in methanol at 70°C Stir the reaction for 6 hours, filter after the reaction, wash with methanol for 5 times, and then dry it in vacuum at 60°C to obtain; chlorine balls and diethanolamine, N,N-dioctylamine, N-methylimidazole, The mass ratio of alkyd anhydride is 10:15:30:8:12.
[0044] In this embodiment, the method for recovering rhenium from the leach solution of arsenic sulfide slag using the above-mentioned composite amine-based weakly basic anion exchange resin comprises the following steps:
[0045] 1) Take 1L of the above compound amine-based weakly basic anion exchange resin and put them into 4 ion exchange columns connected in series (...
Embodiment 3
[0049] The composite amine-based weakly basic anion exchange resin of the present embodiment is prepared by a preparation method comprising the following steps:
[0050] Mix chloromethylated polystyrene-divinylbenzene cross-linked resin (chlorine balls) with diethanolamine, N,N-dioctylamine, N-methylimidazole, and diglycolic anhydride in methanol at 80°C Stir the reaction for 6 hours, filter after the reaction, wash with methanol for 4 times, and then dry it in vacuum at 65°C to obtain; The mass ratio of alkyd anhydride is 10:20:30:15:8.
[0051] In this embodiment, the method for recovering rhenium from the leach solution of arsenic sulfide slag using the above-mentioned composite amine-based weakly basic anion exchange resin comprises the following steps:
[0052] 1) Take 1L of the above composite amine-based weakly basic anion exchange resin and put them into 4 ion exchange columns connected in series (each column is filled with 0.25L resin), and first soak it in 2L of 0.6...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com