Preparation method of key intermediate of Pimobendan
A technology for pimobendan and intermediates, applied in the field of preparation of 4,5-dihydro-5-methyl-6--3 pyridazinone, which can solve the problem of harsh production conditions, difficulty in realizing industrialized production, and many steps and other problems, to achieve the effects of less environmental pollution, easy industrial scale-up, and fewer reaction steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
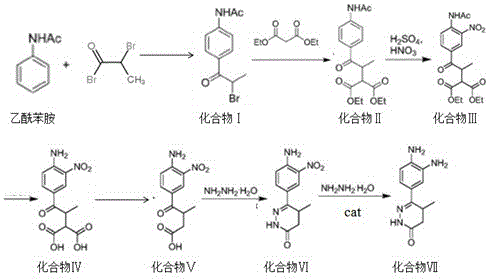
[0042] A preparation method of pimobendan key intermediate, comprising the following steps:
[0043] (i) Synthesis of compound Ⅰ
[0044] In an organic solvent, using acetanilide and 2-bromopropionyl bromide as raw materials, under the action of a Lewis acid catalyst, compound I, i.e. N-(4-(2-bromopropionyl)phenyl)acetamide, is generated;
[0045] (ii) Compound I undergoes a nucleophilic substitution reaction to generate Compound II
[0046] Compound I undergoes a nucleophilic substitution reaction with diethyl malonate in a reaction solvent under the action of a base to generate compound II, namely 2-ethoxycarbonyl-3-(4-acetamidobenzoyl)-butyric acid ethyl ester;
[0047] (Ⅲ) Compound II generates compound III through nitration reaction
[0048] Compound II and mixed acid generate compound III through nitration reaction, that is, ethyl 2-ethoxycarbonyl-3-(3-nitro-4-acetamidobenzoyl)-butyrate;
[0049] (iv) Compound Ⅲ is hydrolyzed to generate compound Ⅳ
[0050] Compound...
Embodiment 1
[0067] (i) Stir 100g of acetanilide and 150g of 2-bromopropionyl bromide into 3L of DMSO solvent, add 60g of ferric chloride catalyst, keep the reaction temperature at -15~-10℃, and stir for 2h~3h , slowly poured the reaction solution into crushed ice, stirred to precipitate a solid and then filtered it. The filter cake was washed with water and dried to obtain 190 g of compound I with a purity of 97% and a melting point of 122°.
[0068] (ii) 100g of compound I, 35g of potassium tert-butoxide, and 120g of diethyl malonate were added to the DMA solution under stirring, and the reaction temperature was kept at 70°C~75°C until TLC traced the disappearance of the raw materials. After the reaction was completed, the system was slowly poured into 1L of ice water to quench, stirred until solid precipitated, filtered and dried to obtain 121 g of Compound II with a purity of 95%.
[0069] (Ⅲ) Mix 600ml of concentrated sulfuric acid and 300ml of concentrated nitric acid and lower the t...
Embodiment 2
[0075] (i) Stir 100g of acetanilide and 200g of 2-bromopropionyl bromide into 2L of dichloromethane solvent, add 200g of zinc chloride catalyst, keep the reaction temperature at -20°C~-30°C, and stir for 1h After ~2h, the reaction solution was slowly poured into crushed ice, the solid was precipitated under stirring, and then filtered. The filter cake was washed with water and dried to obtain 195g of compound I with a purity of 96% and a melting point of 122°.
[0076] (ii) Add 100 g of compound I, 30 g of sodium ethoxide, and 100 g of diethyl malonate into the THF solution under stirring, and keep the reaction temperature at 50°C~55°C until TLC traces the disappearance of the raw materials. After the reaction was completed, the system was slowly poured into 1L of ice water to quench, stirred until solid precipitated, filtered and dried to obtain 120 g of Compound II with a purity of 95%.
[0077] (Ⅲ) Mix 300ml of concentrated sulfuric acid and 900ml of concentrated nitric aci...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com