Charge reversible ion exchange resins, chromatography column, method, and system thereof
A technology of ion-exchange chromatography and ion-exchange groups, which is applied in the direction of ion exchange, anion exchange, and cation exchange materials, and can solve problems such as difficult removal
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1-DV
[0103] Example 1 - DVB / EVB particles
[0104] This example describes the preparation of resin carrier particles based on divinylbenzene and ethylvinylbenzene, known as DVB particles. A solution of 20 grams of 75% benzoyl peroxide in a mixture of 230 grams (g) of divinylbenzene (actually 55% divinylbenzene) and 230 grams of ethylvinylbenzene can be added as fine droplets The form was dispersed in 1600 mL of an aqueous solution containing water and 8 grams of polyvinyl alcohol (Polysciences Inc. Cat. No. 4398, 125,000 g / mole, 88% hydrolyzed). The entire mixture can be shielded from air by maintaining a nitrogen atmosphere within the reaction vessel. The mixture can be heated to 80°C and held at this temperature for twenty hours, during which time polymerization occurs. The liquid can be drained from the resin pellets and then can be washed with water to remove water soluble products, resulting in a white opaque polymer in the form of spherical particles.
example 2
[0105] Example 2-Vinylbenzyldimethylglycine
[0106] This example describes the synthesis of hydrolyzed dimethylglycine ethyl ester (DMGEE) quaternized with vinylbenzyl chloride (VBC), a monomer with tunable ionization states and known as vinylbenzyldimethyl Glycine (see Figure 9 ). 9.955 g H 2 O was added to tall vials (~1" diameter x 5" height). Subsequently, a first portion of 1.962 grams of DMGEE was added to the tall vial and then stirred at room temperature until homogeneous. 10.119 grams of VBC was added to the tall vial and the tall vial was then placed on the stirrer set to medium speed. After 4 hours and 44 minutes, a second portion of 2.125 grams of DMGEE was added to the stirred mixture. About 18 hours after the last addition, a third portion of 2.020 grams of DMGEE was added to the stirred mixture. About 9 hours after the last addition, a fourth portion of 2.163 grams of DMGEE was added to the stirred mixture. About 5 days after the last addition, a fifth ...
example 3
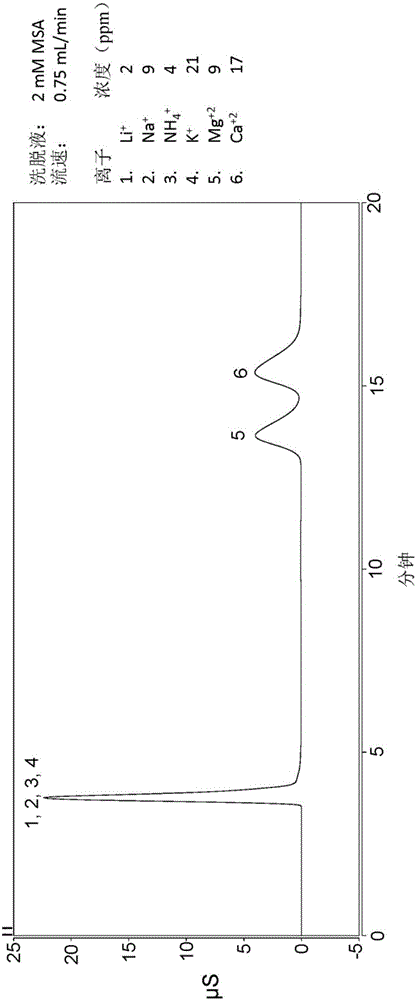
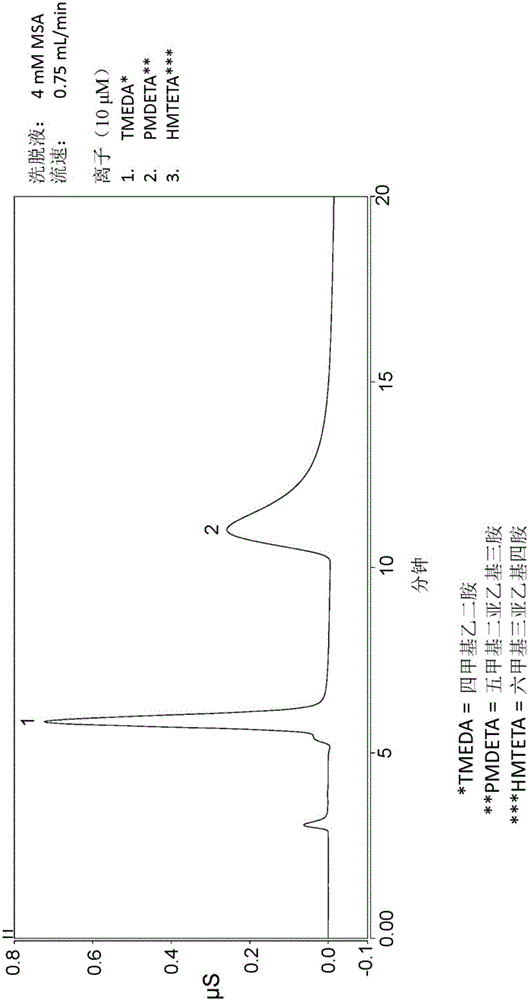
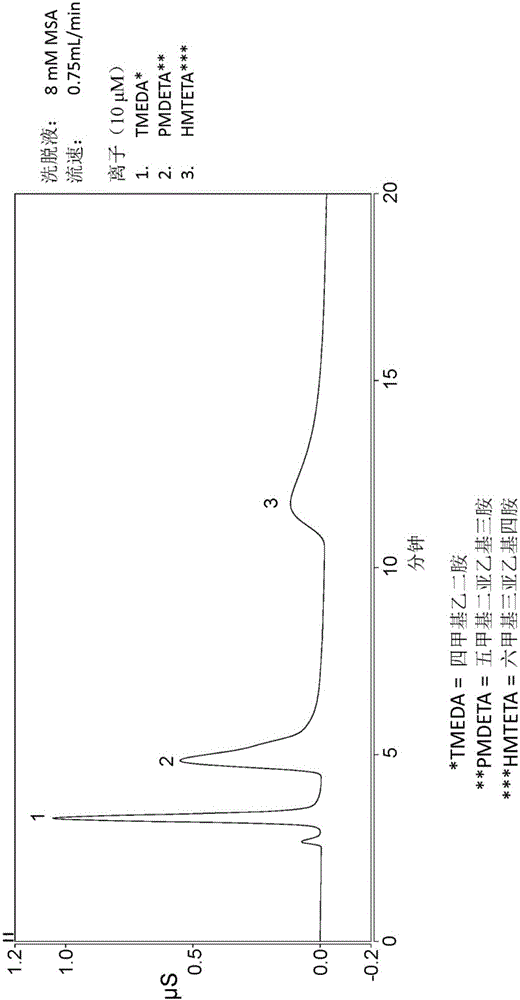
[0108] This example describes the grafting of copolymers to DVB particles (see Figure 10 ) and subsequently packed into the column. 4.007 g of DVB granules from Example 1, 0.613 g of sodium styrene sulfonate, 0.326 g of trimethylvinylbenzyl ammonium chloride, 1.008 g of VBC quaternized hydrolyzed dimethylglycine ethyl Ester, 0.347 grams of V50 initiator (a water-soluble cationic azo initiator, which is available from Wako Specialty Chemicals), 0.515 grams of 2M HNO 3 and 5.096 g H 2 O combined in a container and shaken vigorously to homogenize the slurry. Subsequently, the mixture was sonicated for 2 minutes and the container was then placed on a rotating drum in a 62°C oven for 4.75 days. The resin forms in the container as a dry solid mass. Add water to the container and sonicate to remove resin. Additionally, 20 mL of 1M sodium acetate was added to the vessel and sonicated to remove any remaining resin. Transfer the sonication liquid to the sample cup containing the ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com