Preparation of magnetic nano-drug carrier and method for using magnetic nano-drug carrier to load doxorubicin hydrochloride
A magnetic nano-drug technology, which is applied in the direction of medical formula, drug combination, drug delivery, etc., can solve the problems of poor therapeutic effect and high toxicity, and achieve the effects of low toxic and side effects, strong superparamagnetism, and easy preparation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
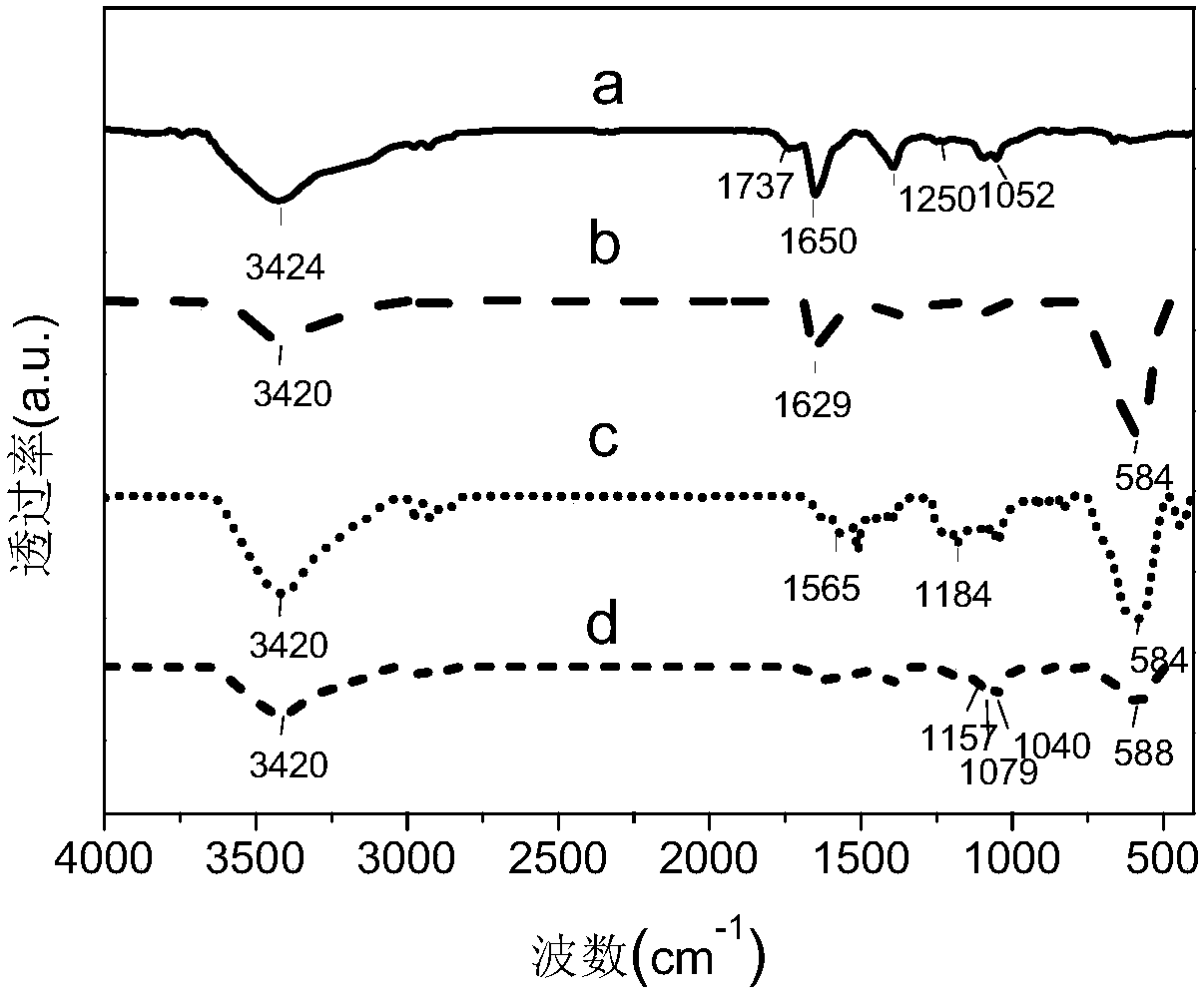
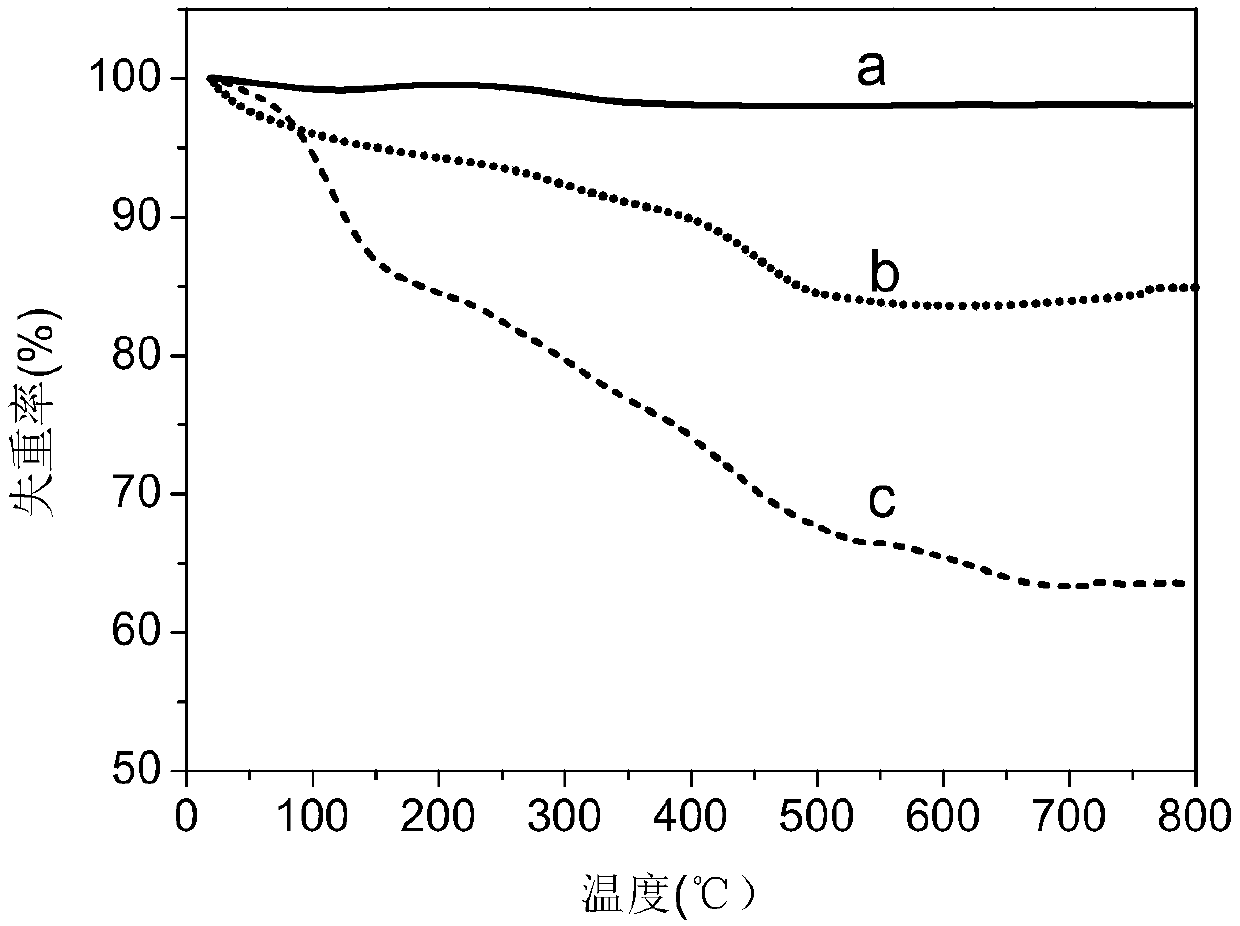
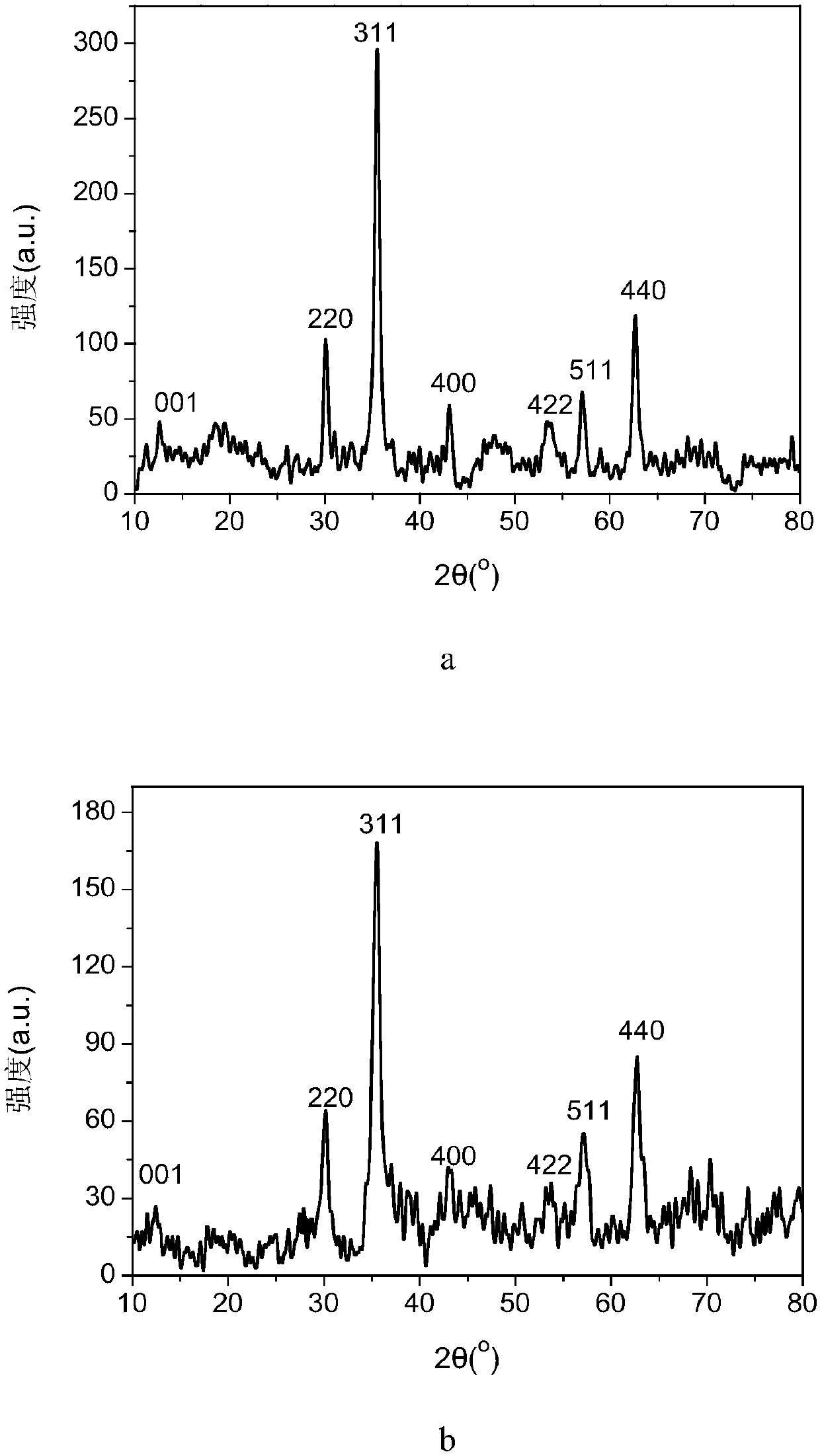
Image
Examples
Embodiment 1
[0041] A preparation method of a magnetic nano-medicine carrier in this embodiment comprises the following steps:
[0042] 1) Preparation of cyclodextrin-hyaluronic acid supramolecular polymer (β-CD-HA):
[0043] First, 18g of β-cyclodextrin (β-CD) and 11.06g of p-toluenesulfonyl chloride (TsCl) were slowly added to 120mL of pyridine solvent and stirred, and reacted at room temperature for 6h; the pyridine solvent was removed by rotary evaporation, and then recrystallized in water 1. Wash and purify with cold acetone to obtain the precipitate mono-6-deoxy-6-(p-toluenesulfonyl)-β-cyclodextrin (M-6-O-Ts-β-CD);
[0044] Then 5 g of mono-6-deoxy-6-(p-toluenesulfonyl)-β-cyclodextrin and 10.55 g of ethylenediamine (EDA) were dissolved in 25 mL of N-N-dimethylformamide (DMF), and the The reaction was carried out under magnetic stirring at 80°C for 18 hours in the environment. After the reaction was completed, the mixture was cooled to room temperature, then washed with cold acetone ...
Embodiment 2
[0056] A preparation method of a magnetic nano-medicine carrier in this embodiment comprises the following steps:
[0057] 1) Preparation of cyclodextrin-hyaluronic acid supramolecular polymer (β-CD-HA):
[0058] First, 17g of β-cyclodextrin (β-CD) and 11g of p-toluenesulfonyl chloride (TsCl) were slowly added into 100mL of pyridine solvent and stirred, and reacted at room temperature for 5h; the pyridine solvent was removed by rotary evaporation, and then recrystallized in water, Wash and purify with cold acetone to obtain the precipitate mono-6-deoxy-6-(p-toluenesulfonyl)-β-cyclodextrin (M-6-O-Ts-β-CD);
[0059] Then 4 g of mono-6-deoxy-6-(p-toluenesulfonyl)-β-cyclodextrin and 10 g of ethylenediamine (EDA) were dissolved in 20 mL of N-N-dimethylformamide (DMF), and the The reaction was carried out under magnetic stirring at 80°C for 18 hours in the environment. After the reaction was completed, the mixture was cooled to room temperature, then washed with cold acetone three ...
Embodiment 3
[0071] A preparation method of a magnetic nano-medicine carrier in this embodiment comprises the following steps:
[0072] 1) Preparation of cyclodextrin-hyaluronic acid supramolecular polymer (β-CD-HA):
[0073] First, 19g of β-cyclodextrin (β-CD) and 12g of p-toluenesulfonyl chloride (TsCl) were slowly added into 150mL of pyridine solvent and stirred, and reacted at room temperature for 8h; the pyridine solvent was removed by rotary evaporation, and then recrystallized in water, Wash and purify with cold acetone to obtain the precipitate mono-6-deoxy-6-(p-toluenesulfonyl)-β-cyclodextrin (M-6-O-Ts-β-CD);
[0074]Then 6 g of mono-6-deoxy-6-(p-toluenesulfonyl)-β-cyclodextrin (M-6-O-Ts-β-CD) and 12 g of ethylenediamine (EDA) were dissolved in 30 mL of N-N-di Methylformamide (DMF), and magnetically stirred at 80°C for 24 hours in a nitrogen atmosphere. After the reaction was completed, the mixture was cooled to room temperature, and then washed with cold acetone for 5 times to r...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com