Method for preparing oxygen-vacancy-containing strontium titanate photocatalyst
A catalyst, strontium titanate technology, applied in the field of preparation of strontium titanate hydrogen photocatalyst, can solve the problems of limited practical application and high cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] (1) Solvothermal preparation of SrTiO 3 .
[0024] Weigh 10mmol tetra-n-butyl titanate and dissolve it in 50mL ethylene glycol, stir it magnetically for 1h, and record it as solution A; weigh 10mmol strontium nitrate and dissolve it in 10mL H 2 O is recorded as solution B; solution B was slowly added dropwise to solution A, and continued to stir for 30 minutes to obtain a colloidal mixture C; slowly dripped 10 mL of NaOH aqueous solution (5.0 mol / L) into the colloidal mixture C, and continued to stir for 30 minutes to obtain a mixed solution d. Transfer the mixed solution D into a 100mL polytetrafluoroethylene liner, react in an autoclave at 180°C for 16h, wash with distilled water and ethanol several times, vacuum dry at 60°C, and grind to obtain SrTiO 3 .
[0025] (2) SrTiO 3 Photoreduction supported Pt (0.50wt%).
[0026] Weigh SrTiO 3 Sample 1.0 g, measure H 2 PtCl 6 Solution 13.3mL (1.93×10 -3 mol / L, Pt load 0.50wt%), added to 20mL of absolute ethanol and...
Embodiment 2
[0036] (1) SrTiO 3 The preparation is the same as in Example 1 step (1).
[0037] (2) SrTiO 3 Photoreduction supported Pt (1.0wt%).
[0038] Weigh SrTiO 3 Sample 1.0 g, measure H 2 PtCl 6 Solution 26.6mL (1.93×10 -3 mol / L, Pt load 1.0wt%), added to 20mL of absolute ethanol and ultrasonically dispersed for 20min, then irradiated with ultraviolet high-pressure mercury lamp for 2h under the protection of nitrogen, washed several times with distilled water and ethanol, vacuum-dried at 60°C, and ground to obtain Photoreduction of Pt / SrTiO with 1.0wt% Pt loading 3 .
[0039] (3)H 2 / N 2 Preparation of Pt / SrTiO by Mixed Gas Calcination 3-x Same as step (3) of Example 1.
[0040] (4) The photocatalytic hydrogen production performance test is the same as the step (4) of Example 1.
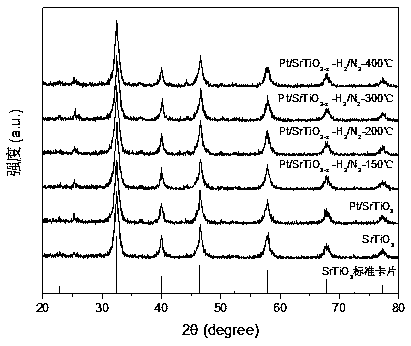
[0041] Figure 5 for H 2 / N 2 Pt / SrTiO prepared by calcination at different temperatures under mixed gas atmosphere 3-x XRD pattern. From Figure 5 It can be seen that strontium titanate ...
Embodiment 3
[0044] (1) SrTiO 3 The preparation is the same as in Example 1 step (1).
[0045] (2) NaBH 4 Chemical reduction of loaded Pt (0.50wt%).
[0046] Weigh SrTiO 3 Sample 1.0 g, measure H 2 PtCl 6 Solution 13.3mL (1.93×10 -3 mol / L, Pt loading 0.50wt%), added to 50mL distilled water and ultrasonically dispersed for 20min. Weigh NaBH 4 9.7 mg dissolved in 10mL distilled water (NaBH 4 with H 2 PtCl 6 The molar ratio is 10:1), slowly added dropwise to the above SrTiO 3 with H 2 PtCl 6 The mixed solution was magnetically stirred at room temperature for 4 h. Wash several times with distilled water and ethanol, vacuum dry at 60°C, and grind to obtain NaBH 4 Chemical reduction of Pt / SrTiO with Pt loading of 0.50wt% 3 .
[0047] (3)H 2 / N 2 Preparation of Pt / SrTiO by Mixed Gas Calcination 3-x With embodiment 1 step 3, calcining temperature is 200 ℃
[0048] (4) The photocatalytic hydrogen production performance test is the same as the step (4) of Example 1.
[0049] ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com