A kind of preparation method and application of ruthenium/nickel alloy nano catalyst
A nano-catalyst and catalyst technology, applied in the field of nano-catalyst research, can solve problems such as limited research work, and achieve the effects of simple production process requirements, mild reaction conditions, and avoiding high-temperature and high-pressure reactions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] 1. Preparation of catalyst:
[0023] Using ruthenium trichloride and nickel acetate as raw materials and hydrogen peroxide as reducing agent, the ruthenium / nickel alloy nanocatalyst was prepared in the presence of organic modifiers.
[0024] When the molar ratio of ruthenium and nickel was 0.05:1, the ruthenium / nickel alloy nanocatalyst (Ru 0.05 Ni) preparation: take 0.1720g of Ru (NO 3 ) 2 2H 2 O, 4.9768g of Ni (NO 3 ) 2 and 0.4977g of KH560 silane coupling agent were dissolved in 40mL, 70mL and 10mL of absolute ethanol respectively by ultrasonic dispersion, and stirred and mixed at 50°C for 20min. Use 1.0mol / L NaOH ethanol solution to adjust the pH to 10, add 0.75mol / L hydrogen peroxide / lithium aluminum hydride absolute ethanol solution dropwise to the above reaction solution, react at 70°C for 6h, and the product undergoes several times After alcohol washing and vacuum drying, the Ru 0.05 Ni alloy nanocatalyst.
[0025] 2.1-Nitroanthraquinone selective cataly...
Embodiment 2
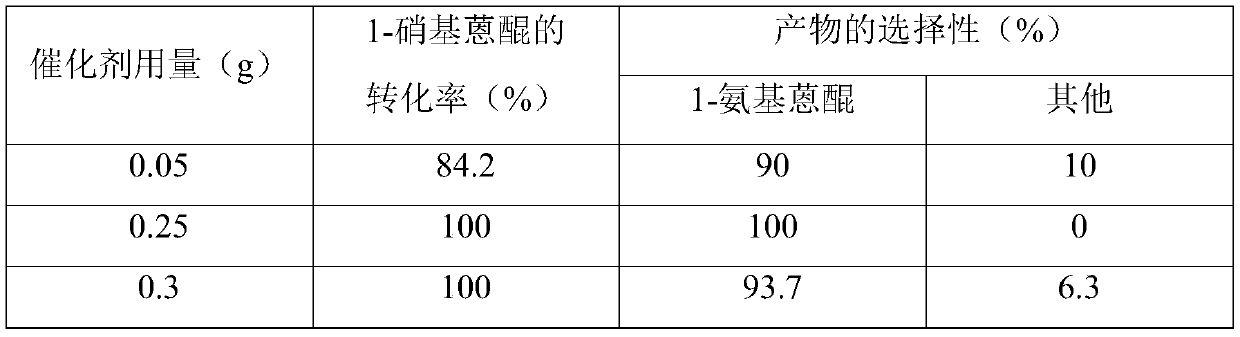
[0028] Same as in Example 1, only changing the amount of catalyst: 0.05g, 0.25g, 0.3g, to carry out the selective hydrogenation reaction of 1-nitroanthraquinone. The product selectivity and raw material conversion rate obtained are shown in Table 1.
[0029] Table 1 at 1.0MPa H 2 The reaction temperature is 120°C, and the reaction is carried out for 5 hours under heat preservation. When the amount of catalyst is different, the selectivity of 1-aminoanthraquinone hydrogenation reaction product 1-aminoanthraquinone and the conversion rate of raw materials are selectively catalyzed by ruthenium / nickel alloy nanocatalysts.
[0030]
Embodiment 3
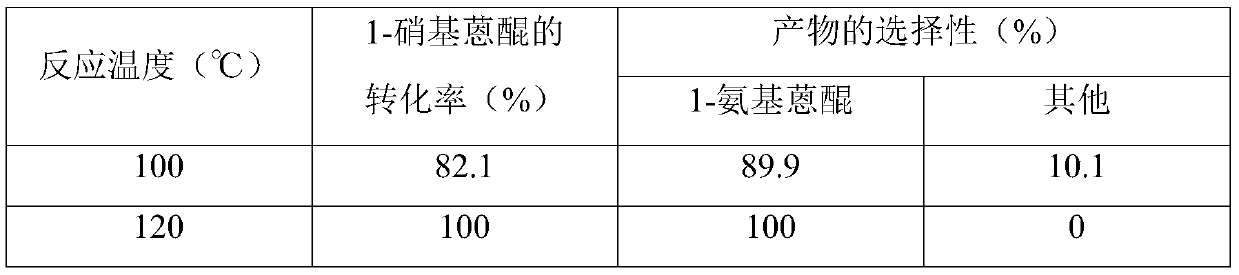
[0032] Same as in Example 1, only changing the temperature of the reactor to 100°C, 120°C, and 140°C to carry out the selective hydrogenation reaction of 1-nitroanthraquinone. The resulting product selectivity and feedstock conversion are shown in Table 2.
[0033] Table 2 at 1.0MPa H 2 Under pressure, when the amount of catalyst is 0.25g, under different reaction temperatures, under heat preservation for 5 hours, the ruthenium / nickel alloy nano-catalyst selectively catalyzes the selectivity of 1-aminoanthraquinone hydrogenation reaction product of 1-nitroanthraquinone and the conversion of raw materials Rate
[0034]
[0035]
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com