Method for synthesizing nanometer kaolin by using muscovite powder
A technology of muscovite powder and kaolinite, which is applied in the preparation of alkali metal nitrate, aluminum silicate, silicate, etc., can solve the problems of complicated production process, expensive potassium chloride, dangerous and explosive ammonium nitrate, etc. Achieve high resource utilization, good economic benefits and application prospects, and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
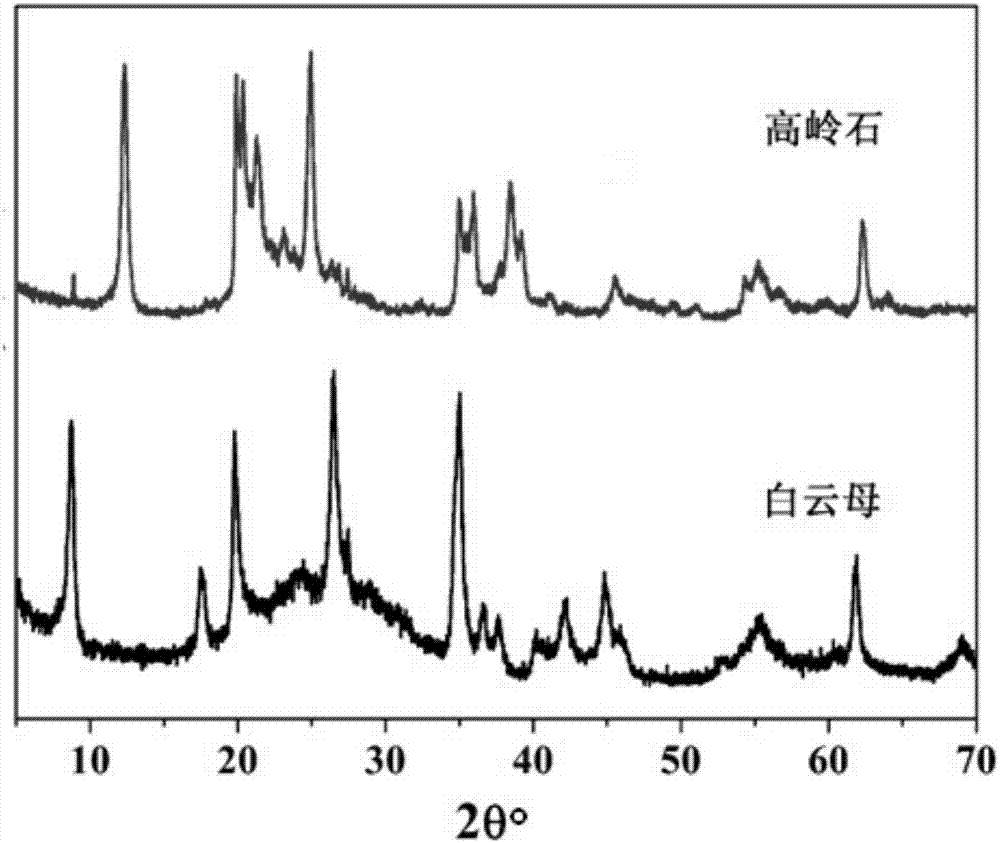
[0026] Accurately take by weighing 6 g of muscovite powder (chemical composition analysis results are shown in Table 1), first the mass concentration of nitric acid is 65 ~ 68wt% nitric acid diluted with distilled water to a concentration of 1.5mol / kg, the mass concentration of muscovite powder and nitric acid solution Ratio 1:50, mixed and stirred evenly, put into a stainless steel reactor with polytetrafluoroethylene lining, hydrothermally reacted at 250°C for 24 hours, immediately took out the reactor and put it in cold water for rapid cooling, and solidified after the kettle was opened. The liquid is separated, and the filtrate is evaporated and crystallized at 110-120°C to obtain the potassium nitrate product. Test for K in Potassium Nitrate 2 The O content is 45.23%, and the purity of potassium nitrate equivalent is greater than 97%. The dissolution rate of potassium oxide measured by ICP was 98%. The filter cake is washed with distilled water until the pH of the filtr...
Embodiment 2
[0030] Accurately take by weighing 6 g of muscovite powder (chemical composition analysis results are shown in Table 1), first the mass concentration of nitric acid is 65 ~ 68wt% nitric acid diluted with distilled water to a concentration of 0.9mol / kg, the mass concentration of muscovite powder and nitric acid solution Ratio 1:25, mixed and stirred evenly, put into a stainless steel reactor with polytetrafluoroethylene liner, hydrothermally reacted at 250°C for 24 hours, immediately took out the reactor and put it in cold water for rapid cooling, and solidified after opening the kettle. The liquid is separated, and the filtrate is evaporated and crystallized at 110-120°C to obtain potassium nitrate to test the K in potassium nitrate 2 The O content is 45.19%, and the purity of potassium nitrate equivalent is greater than 97%. The dissolution rate of potassium oxide measured by ICP was 98%. The filter cake is washed with distilled water until the pH of the filtrate is 9-10, an...
Embodiment 3
[0032] Accurately take by weighing 6 g of muscovite powder (chemical composition analysis results are shown in Table 1), first the mass concentration of nitric acid is 65 ~ 68wt% nitric acid diluted with distilled water to a concentration of 1.2mol / kg, the mass concentration of muscovite powder and nitric acid solution Ratio 1:25, mixed and stirred evenly, put into a stainless steel reactor with polytetrafluoroethylene liner, hydrothermally reacted at 250°C for 24 hours, immediately took out the reactor and put it in cold water for rapid cooling, and solidified after opening the kettle. The liquid is separated, and the filtrate is evaporated and crystallized at 110-120°C to obtain the potassium nitrate product, and the K in the potassium nitrate is tested. 2 The O content is 45.43%, and the purity of potassium nitrate equivalent is greater than 97%. The dissolution rate of potassium oxide measured by ICP was 98%. The filter cake is washed with distilled water until the pH of ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com