Method for synthesizing trans-4-alkylcyclohexyl benzene structure liquid crystal intermediates and monomers
A technology for the synthesis of cyclohexylbenzene, which is applied to the preparation of heterocyclic compounds, chemical instruments and methods, magnesium organic compounds, etc., can solve the problems of limited conversion rate and achieve the effect of avoiding low yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
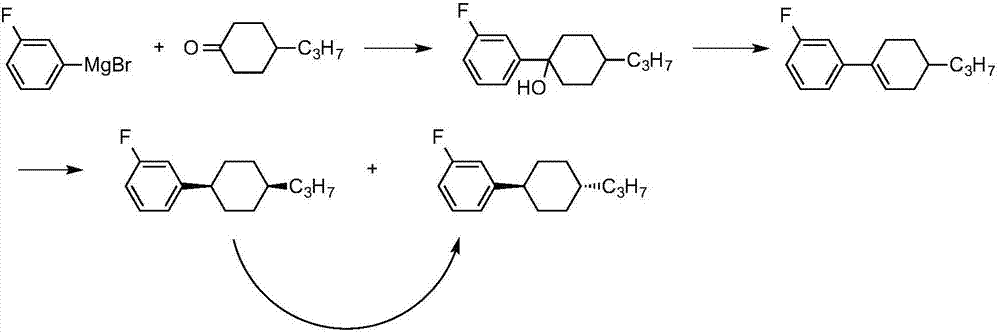
[0034] 1-fluoro-3-(trans-4-propylcyclohexyl)benzene Synthesis
[0035] In a 500ml three-necked flask, add 5.9g (0.24mol) of magnesium chips and 20ml of THF, and drop in a solution made of 38.5g (0.22mol) of 3-fluorobromobenzene and 140ml of THF to prepare 3-fluorophenylmagnesium bromide. Raise the temperature to 60°C, keep a stable reaction, add dropwise a solution of 31.2g (0.2mol) of 1,4-cyclohexanedione monoethylene glycol ketal and 120ml of toluene, keep stirring for 3 hours after dropping, and cool down to 0°C. Slowly add 200ml of 2N hydrochloric acid while keeping it below 10°C, let stand to separate the upper organic layer, extract the water layer with 120ml of toluene once, combine the organic phases, evaporate the solvent to 100°C, add 1g of p-toluenesulfonic acid, 5g of ethylene glycol, Reflux to separate water for 5h until no water comes out. The reaction solution was washed with aqueous sodium bicarbonate until neutral, and the toluene was evaporated to dryness ...
Embodiment 2
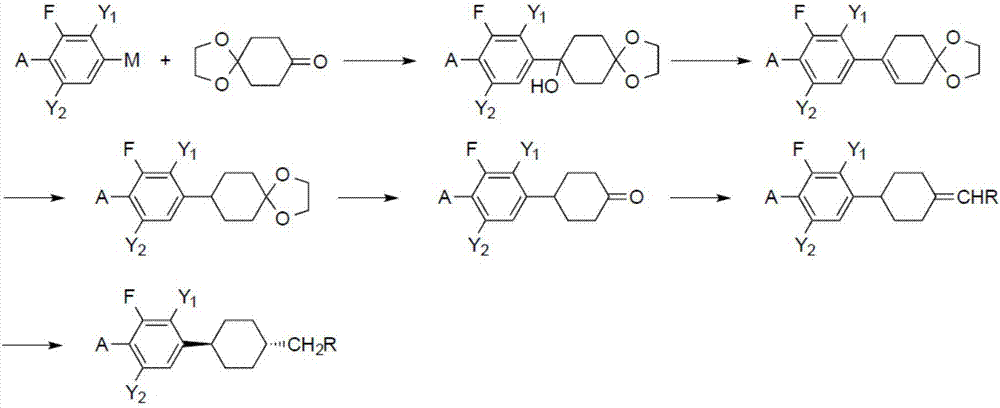
[0043] 1,3-Difluoro-5-(trans-4-pentylcyclohexyl)benzene Synthesis
[0044] In a 500ml three-necked flask, add 5.9g (0.24mol) of magnesium chips and 20ml of THF, and drop in a solution made of 42.5g (0.22mol) of 3,5-difluorobromobenzene and 150ml of THF to prepare 3,5-difluorophenyl magnesium bromide. Raise the temperature to 60°C, keep a stable reaction, add dropwise a solution of 31.2g (0.2mol) of 1,4-cyclohexanedione monoethylene glycol ketal and 120ml of toluene, keep stirring for 3 hours after dropping, and cool down to 0°C. Slowly add 200ml of 2N hydrochloric acid while keeping it below 10°C, let stand to separate the upper organic layer, extract the water layer with 120ml of toluene once, combine the organic phases, evaporate the solvent to 100°C, add 1g of p-toluenesulfonic acid, 5g of ethylene glycol, Reflux to separate water for 5h until no water comes out. The reaction solution was washed with aqueous sodium bicarbonate until neutral, and the toluene was evaporat...
Embodiment 3
[0052] 1-ethoxy-2,3-difluoro-4-(trans-4-propylcyclohexyl)benzene Synthesis
[0053] Into a 500ml three-necked flask, add 170ml of THF and 34.8g (0.22mol) of 2,3-difluorophenetole. Stir and cool down to -78°C, control the temperature below -70°C and add 88ml (0.22mol) of 2.5mol / L butyllithium solution dropwise, and keep warm for 1h after dropping. Keep the temperature controlled below -60°C and add dropwise a solution of 1,4-cyclohexanedione monoethylene glycol ketal 31.2g (0.2mol) and THF 100ml, keep stirring for 1h after dropping, and heat up to 0°C. Slowly add 200ml of 2N hydrochloric acid while keeping below 10°C, let stand to separate the upper organic layer, extract the water layer with 200ml of toluene once, combine the organic phases, evaporate the solvent to 100°C, add 1g of p-toluenesulfonic acid, 5g of ethylene glycol, Reflux to separate water for 5h until no water comes out. The reaction solution was washed with aqueous sodium bicarbonate until neutral, and the ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com