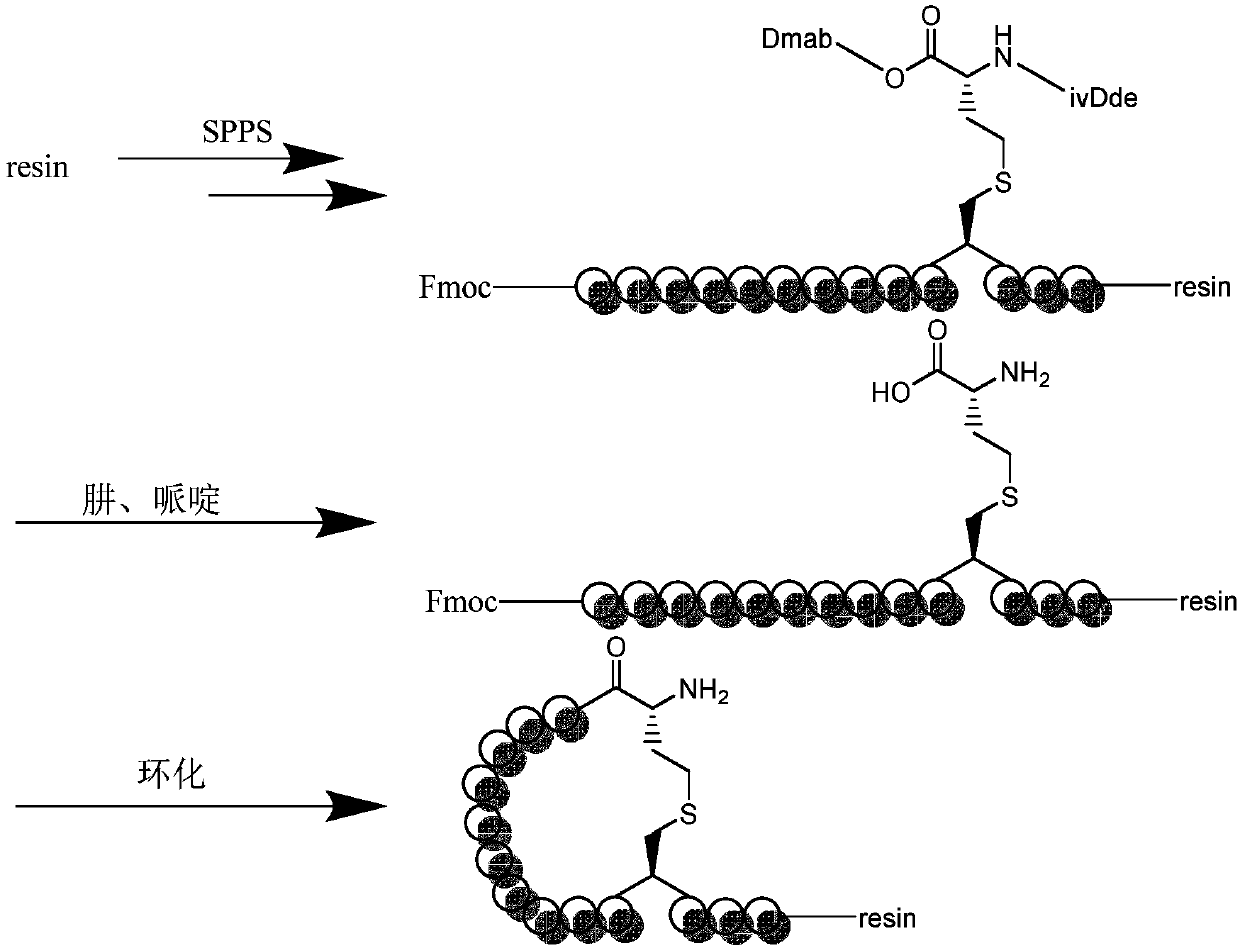
Environmentally-friendly orthogonally protected diaminodiacid compound, and preparation method and application thereof
A technology for diaminodiacids and compounds, which is applied in the field of environmentally friendly orthogonally protected diaminodiacid compounds, can solve problems such as self-activity influence and environmental pollution, and achieve the effect of increasing species
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0078] Example 1: Preparation of N-fluorenylmethoxycarbonyl-S-trityl-L-cysteine allyl ester
[0079]
[0080] Dissolve 8.4 mmol of cysteine or its derivatives (represented by Fmoc-Cys (Trt) or side chain Trt-protected cysteine) in 20 ml of DMF, then add 10 mmol of sodium bicarbonate and 10 mmol of allyl bromide and stir React overnight; add ethyl acetate after the reaction is complete, then wash the organic phase with saturated brine, add anhydrous sodium sulfate to the resulting organic phase to dry, filter and concentrate the organic phase, and separate by column chromatography (petroleum ether / ethyl acetate=5 : 1, v / v) to obtain 4.7mmol intermediate product I.
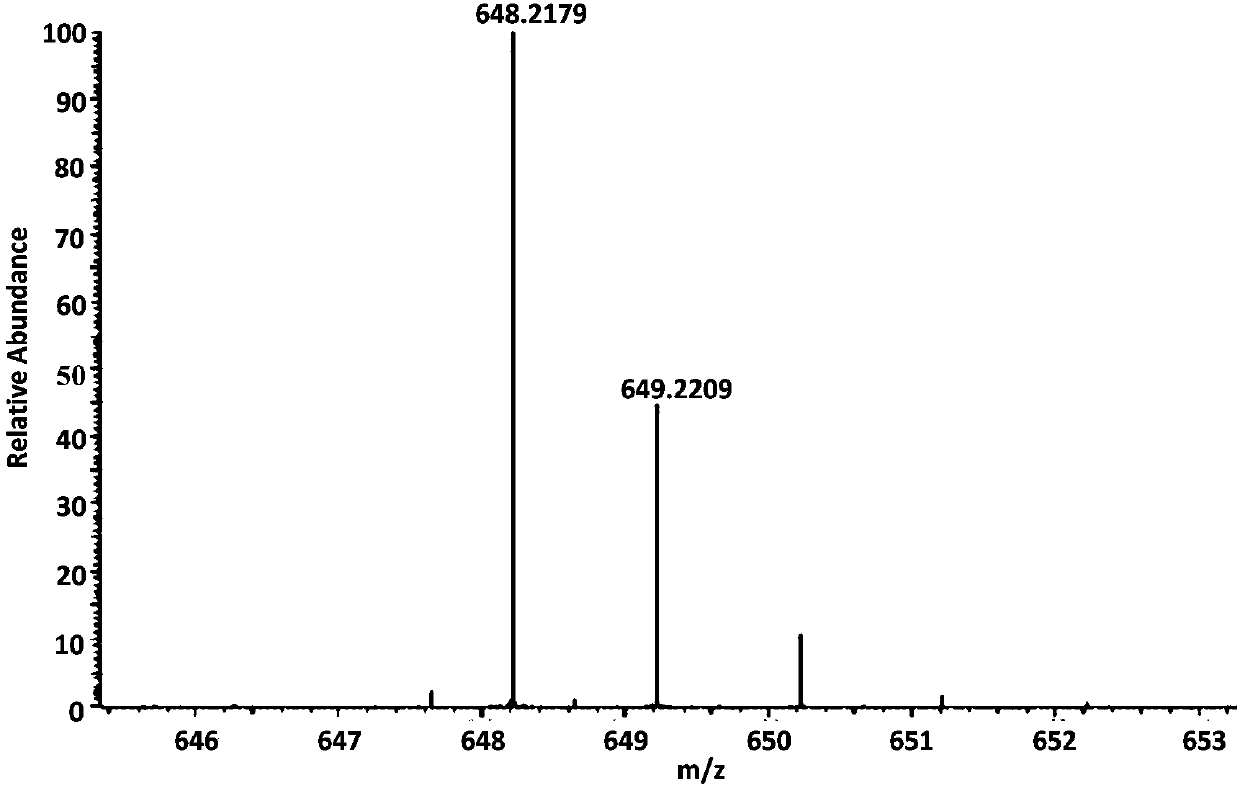
[0081] ESI-MS (positive): 648.2179 (observed, M+Na); 625.2287 (calculated, M).
[0082] 1 H NMR (400MHz, CDCl 3 )δ7.76(dd,J=7.4,3.7Hz,2H),7.60(dd,J=7.2,2.6Hz,2H), 7.40(dd,J=5.4,3.4Hz,8H),7.29(dd,J =12.8,7.3Hz,6H),7.24(d,J=2.2Hz,2H),7.22–7.17 (m,3H),5.87(dq,J=10.9,5.7Hz,1H),5.35–5.21(m, 3H), 4.65–4.53(m, ...
Embodiment 2
[0083] Embodiment 2: Preparation of N-tert-butoxycarbonyl-L-homoserine tert-butyl ester
[0084]
[0085] Dissolve 10mmol homoserine and 40mmol sodium bicarbonate in 30ml water, then slowly add 10ml dioxane and stir for 5min, add 15mmol Boc anhydride in ice bath, then stir and react at room temperature for 12h; after the reaction, wash the reaction solution with ether Twice, use 2M hydrochloric acid to adjust the pH of the aqueous phase to 2, extract the aqueous phase with ethyl acetate and combine the organic phase, wash the organic phase with saturated brine, dry over anhydrous sodium sulfate and remove the solvent by rotary evaporation to obtain the N-terminal Boc-protected Homoserine residues;
[0086] Dissolve the obtained N-terminal Boc-protected homoserine residue, 10mmol TEBAC, and 54mmol potassium carbonate in 25ml DMF, then slowly add 90mmol tert-butyl bromide and stir, and react for 48h at 50°C without light; after the reaction, wash with 75ml water Dilute the o...
Embodiment 3
[0088] Example 3: Preparation of (2S)-3-bromo-2-(tert-butoxycarbonyl)amino-butyric acid tert-butyl ester
[0089]
[0090]Mix 4mmol of the C-terminal tBu-protected homoserine residue prepared in Example 2 directly with 5mmol of carbon tetrabromide and dissolve in 25ml of dichloromethane solution, slowly add 5mmol of triphenylphosphine and 10ml of dichloromethane dropwise under ice-bath conditions The mixed solution of methane was reacted at room temperature and nitrogen atmosphere for 2h; the reaction mixture was diluted with dichloromethane, washed with saturated brine, dried over anhydrous sodium sulfate, filtered and concentrated to 10ml by rotary evaporation, and the obtained solution was concentrated with a volume ratio of 1: 1 was extracted with ethyl acetate and petroleum ether, and the oxides of phosphorus were precipitated. After the extract was concentrated and dried, it was separated through a silica gel column (the eluent was petroleum ether:ethyl acetate=5:1, v / ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com