Lithium/sodium dual-ion manganese-based oxide positive electrode material and preparation method and application thereof
A technology of manganese-based oxides and positive electrode materials, which is applied in the direction of battery electrodes, electrochemical generators, electrical components, etc., can solve the problems of low initial efficiency, initial charge and discharge efficiency of materials, and cycle stability, etc., to achieve convenient operation, Effect of improving discharge specific capacity and cycle stability, good discharge specific capacity and cycle stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
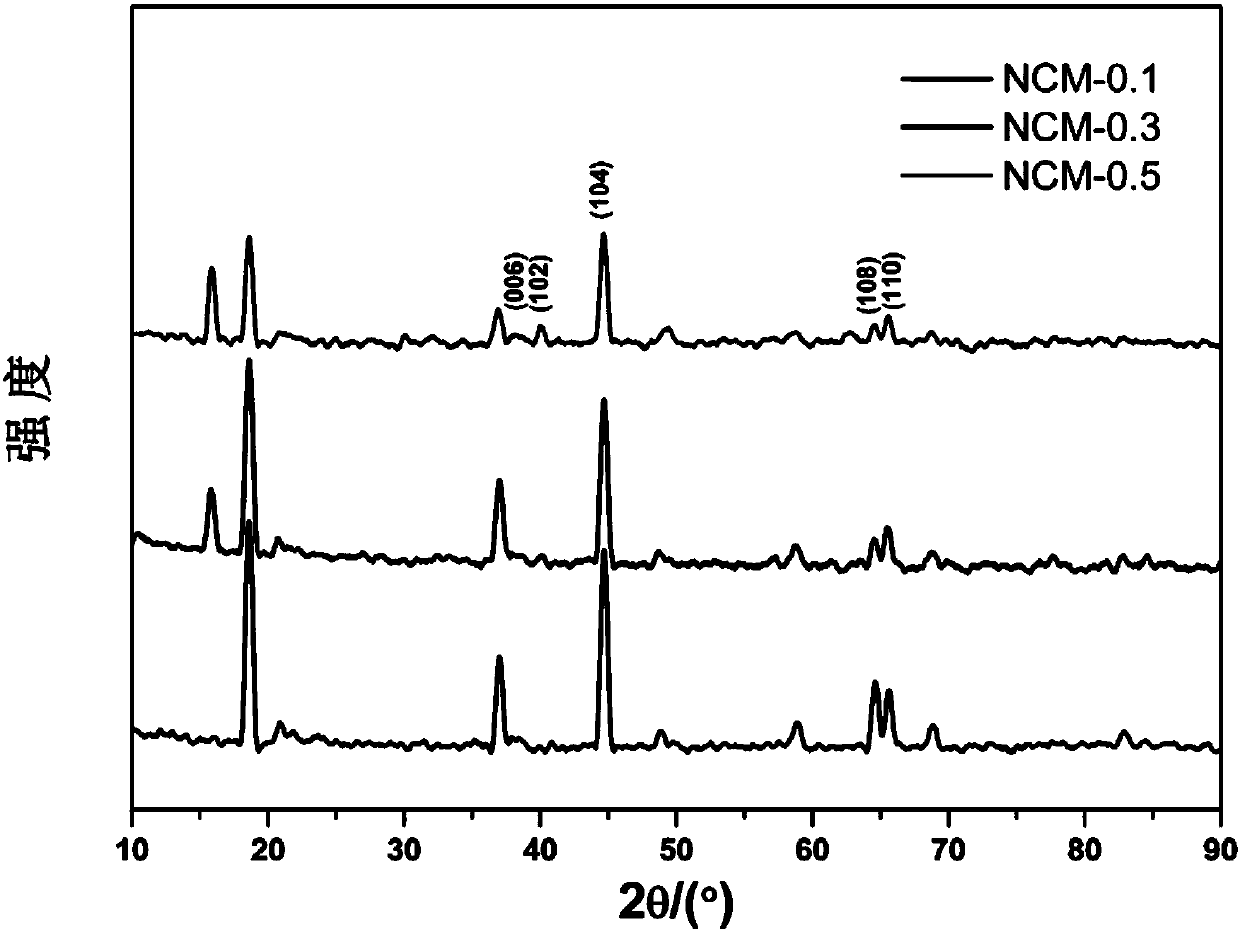
Image
Examples
Embodiment 1
[0071] (1) Disperse 4.09g manganese acetate tetrahydrate, 1.04g nickel acetate tetrahydrate and 1.04g cobalt acetate tetrahydrate in 25mL deionized water to obtain a mixed transition metal salt solution;
[0072] (2) Disperse 5.7g oxalic acid dihydrate in 100mL deionized water to obtain an oxalic acid solution;
[0073] (3) Disperse 2.385 g of anhydrous lithium acetate and 0.269 g of anhydrous sodium acetate in 25 mL of deionized water. Wherein, lithium salt is excessive 5%wt to replenish the lithium volatilized in the calcining process;
[0074] (4) Add the mixed transition metal salt solution in step (1) to the oxalic acid solution in step (2), stir while adding it dropwise to make it react fully, the stirring speed is 800rmp, continue to stir for 2h after the dropwise addition, and stir to make The reaction system is uniformly mixed and dispersed, which is conducive to the full progress of the reaction;
[0075] (5) Add the lithium acetate and sodium acetate solution prep...
Embodiment 2
[0079] (1) Disperse 4.09g manganese acetate tetrahydrate, 1.04g nickel acetate tetrahydrate and 1.04g cobalt acetate tetrahydrate in 25mL deionized water to obtain a mixed transition metal salt solution;
[0080] (2) Disperse 5.7g oxalic acid dihydrate in 100mL deionized water to obtain an oxalic acid solution;
[0081] (3) Disperse 1.95 g of anhydrous lithium acetate and 0.808 g of anhydrous sodium acetate in 25 mL of deionized water. Wherein, lithium salt is excessive 5%wt to replenish the lithium volatilized in the calcining process;
[0082] (4) Add the mixed transition metal salt solution in step (1) to the oxalic acid solution in step (2), and stir while adding it dropwise to make it react fully, the stirring speed is 800rmp, and continue to stir for 2h after the dropwise addition;
[0083] (5) Add the lithium acetate and sodium acetate solution prepared in step (3) to the reaction solution in step (4), stir while adding dropwise to make it evenly mixed, the stirring sp...
Embodiment 3
[0087] (1) Disperse 4.09g manganese acetate tetrahydrate, 1.04g nickel acetate tetrahydrate and 1.04g cobalt acetate tetrahydrate in 25mL deionized water to obtain a mixed transition metal salt solution;
[0088] (2) Disperse 5.7g oxalic acid dihydrate in 100mL deionized water to obtain an oxalic acid solution;
[0089] (3) Disperse 1.517 g of anhydrous lithium acetate and 1.346 g of anhydrous sodium acetate in 25 mL of deionized water. Wherein, lithium salt is excessive 5%wt to replenish the lithium volatilized in the calcining process;
[0090] (4) Add the mixed transition metal salt solution in step (1) to the oxalic acid solution in step (2), and stir while adding it dropwise to make it react fully, the stirring speed is 800rmp, and continue to stir for 2h after the dropwise addition;
[0091] (5) Add the lithium acetate and sodium acetate solution prepared in step (3) to the reaction solution in step (4), stir while adding dropwise to make it evenly mixed, the stirring s...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Charge specific capacity | aaaaa | aaaaa |
| Charge specific capacity | aaaaa | aaaaa |
| Cycle efficiency | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com