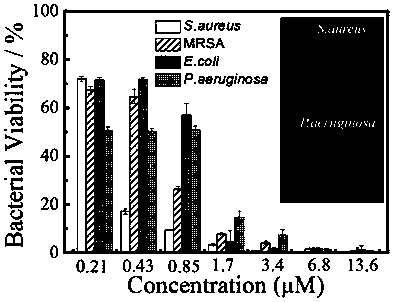
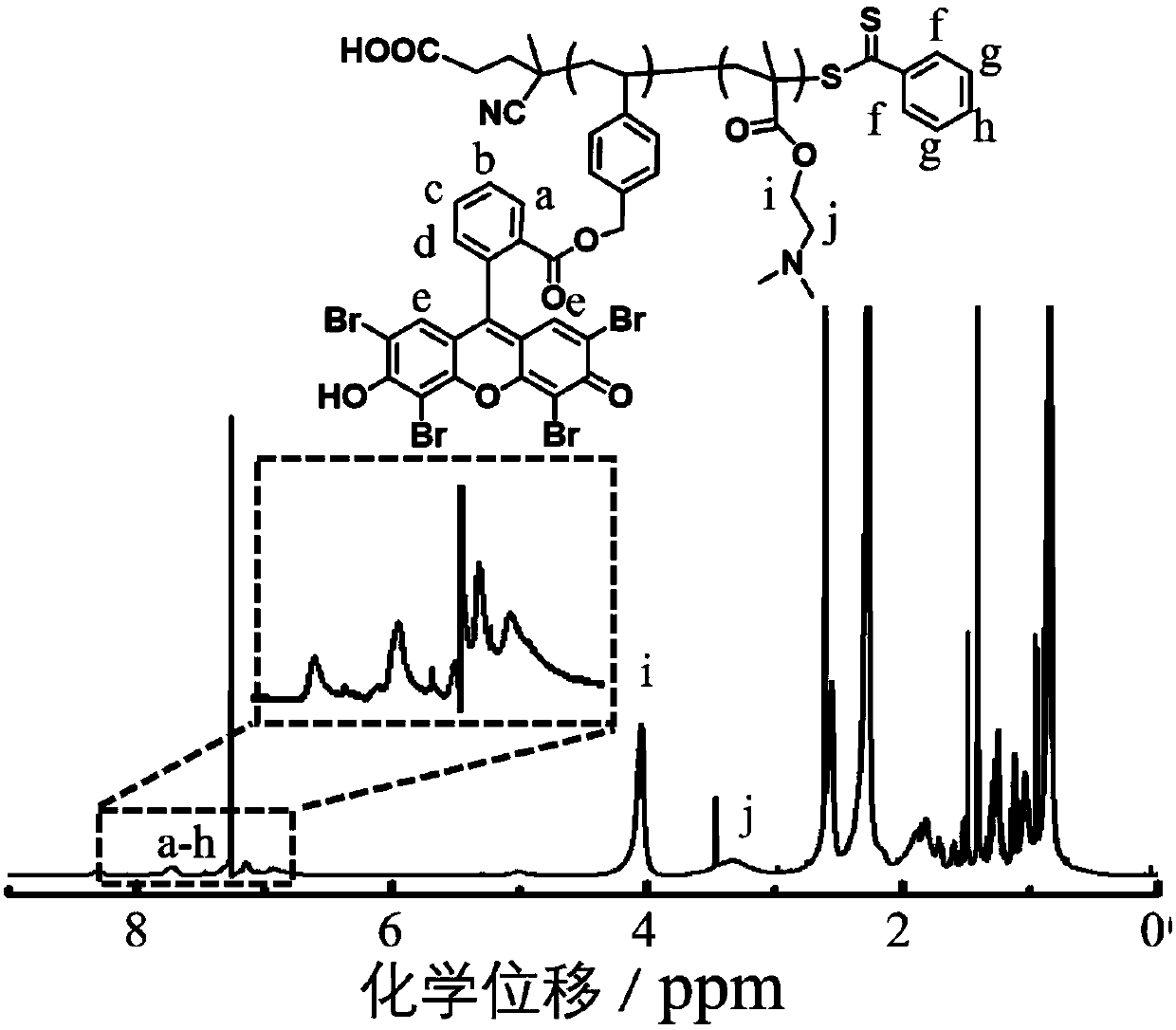
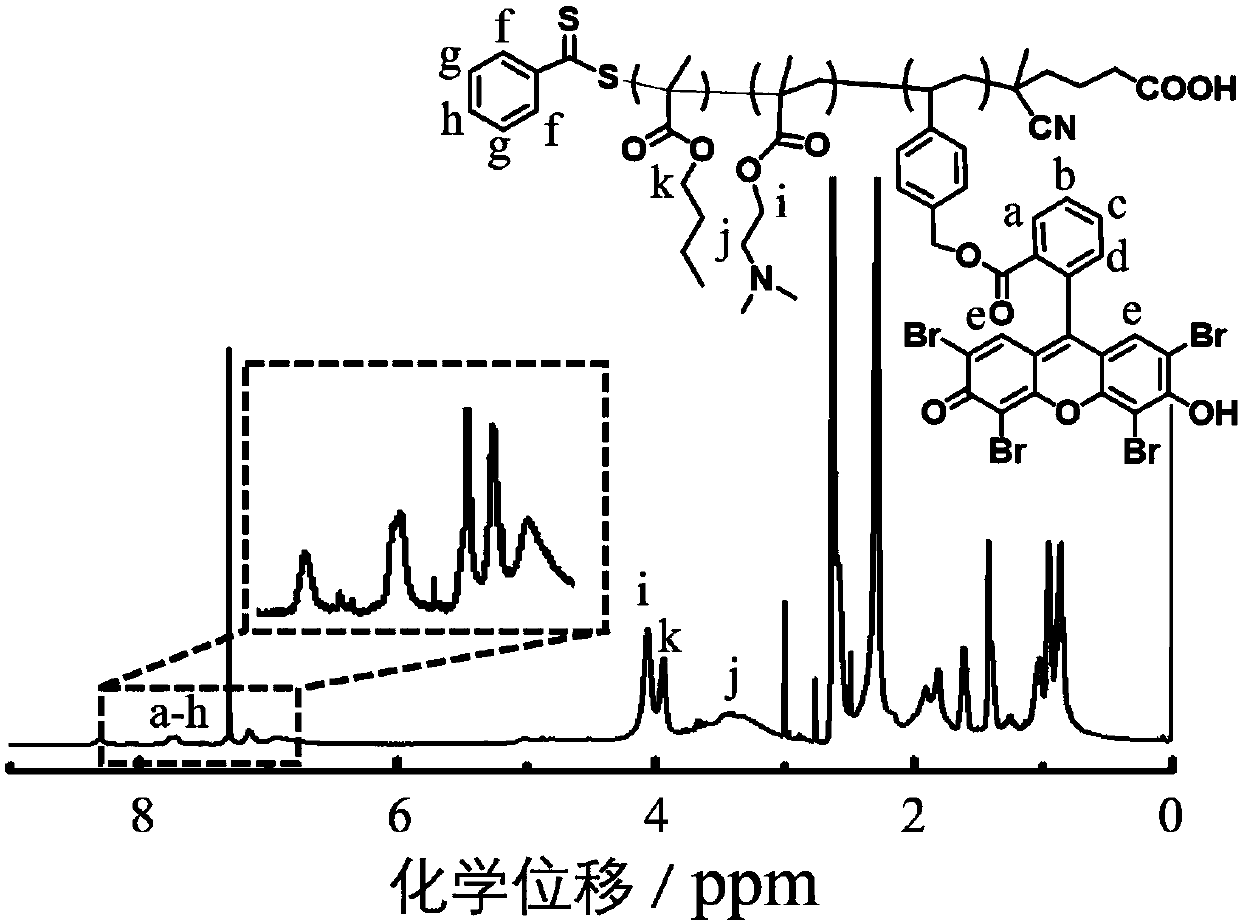
Preparation of bacterium targeting nanoparticles and application of bacterium targeting nanoparticles to bacterium inhibition and killing
A nanoparticle, targeted technology, applied in antibacterial drugs, medical preparations with non-active ingredients, and medical preparations containing active ingredients, etc., can solve the problem of high toxicity of antimicrobial peptides
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0069] Embodiment 1: the preparation of P (DMAEMA-co-EOS) macromolecular chain transfer agent
[0070] With reference to the above reaction formula (a), the small molecule chain transfer agent CTA (13.9 mg, 0.05 mmol), N, N-dimethylaminoethyl methacrylate (DMAEMA, 353.7 mg, 2.25mmol), p-chloromethylstyrene modified eosin (EOS, 191mg, 0.25mmol) and azobisisobutyronitrile AIBN (1.64mg, 0.01mmol) were dissolved in 1.5mL 1,4-bis Oxyhexane. Freeze the ampoule bottle in liquid nitrogen and use an oil pump to pump air, then seal the ampoule bottle, return to room temperature to melt the reaction mixture, and then freeze and pump air again, repeat the freeze-thaw cycle three times, then seal it under vacuum, 70°C After the reaction was stirred for 20 hours, the polymerization reaction was stopped with liquid nitrogen, the reaction bottle was opened, the reaction mixture was precipitated in petroleum ether, centrifuged, then dissolved in dichloromethane and precipitated with a large a...
Embodiment 2
[0072] Embodiment 2: P(DMAEMA 20 -co-EOS 0.8 )-b-PBMA 16 Preparation of Amphiphilic Block Polymers
[0073] With reference to above-mentioned reaction formula (b), the prepared macromolecular chain transfer agent P(DMAEMA of embodiment 1 20 -co-EOS 0.8 ) (302 mg, 0.075 mmol), butyl methacrylate (BMA, 213 mg, 1.5 mmol) and azobisisobutyronitrile AIBN (2.46 mg, 0.015 mmol) were dissolved in 1.1 mL of 1,4-dioxo Hexacyclic and 0.9 mL DMSO. Freeze the ampoule bottle in liquid nitrogen and use an oil pump to pump air, then seal the ampoule bottle, return to room temperature to melt the reaction mixture, and then freeze and pump air again, repeat the freeze-thaw cycle three times, then seal it under vacuum, 70°C After stirring for 19 hours, stop the polymerization reaction with liquid nitrogen, open the reaction bottle, precipitate the reaction mixture in petroleum ether, centrifuge, redissolve in DMF and precipitate with a large amount of petroleum ether, repeat three times, an...
Embodiment 3
[0075] Embodiment 3: (UBI-P(DMAEMA 20 -co-EOS 0.8 )-b-PBMA 16 ) preparation
[0076] With reference to the above reaction formula (c), the amphiphilic block polymer P(DMAEMA prepared in Example 2 20 -co-EOS 0.8 )-b-PBMA 16 (31.5mg, 0.005mmol) was added to a 5mL ampoule and dissolved in 1mLDMF, and 3mL of toluene was added. After the azeotropic removal of water with toluene for 0.5h, 1-(3-dimethylaminopropyl)-3-ethylcarbodiol was added Imine hydrochloride (EDC·HCl, 1.9mg, 0.01mmol) and N-hydroxysuccinimide (1.2mg, 0.01mmol) were sealed and stirred at room temperature for 12h, and the amphiphilic block polymer P(DMAEMA 20 -co-EOS 0.8 )-b-PBMA 16After activation of the terminal carboxyl group, add bacterial targeting peptide (UBI, 6.76mg, 0.004mmol) and seal and stir at room temperature for 24h, then precipitate the reaction mixture in ether, centrifuge, dissolve in DMF and precipitate with a large amount of petroleum ether, Repeated three times, the final product was dri...
PUM
| Property | Measurement | Unit |
|---|---|---|
| The average diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com