Synthesis method for preparing trisubstituted alkenes based on nickel catalysis
A synthesis method and tri-substituted technology are applied in the field of synthesis of single-configuration tri-substituted olefin compounds, which can solve the problems of difficulty in synthesis, limited source of raw materials, and difficulty in separation, and achieve the effects of wide sources and simple feeding methods.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
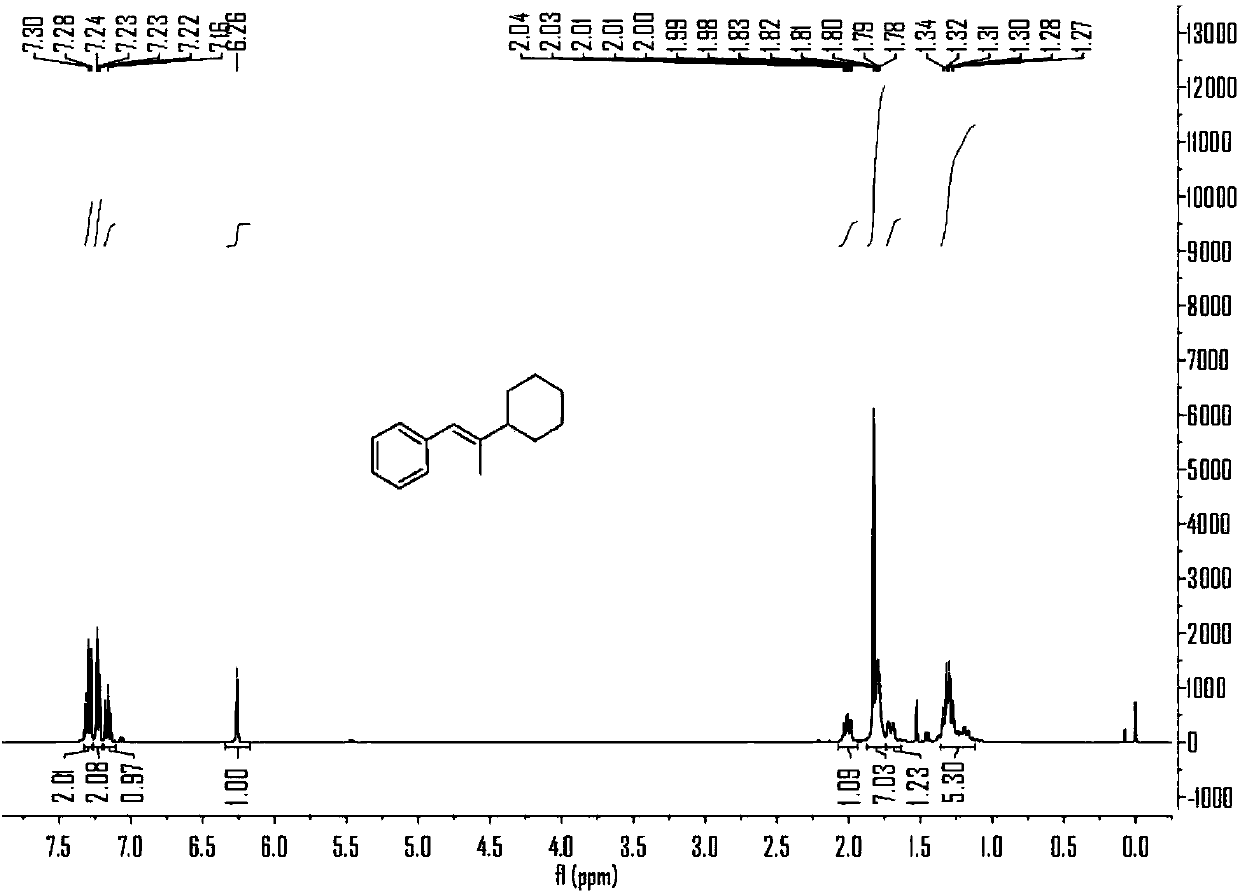
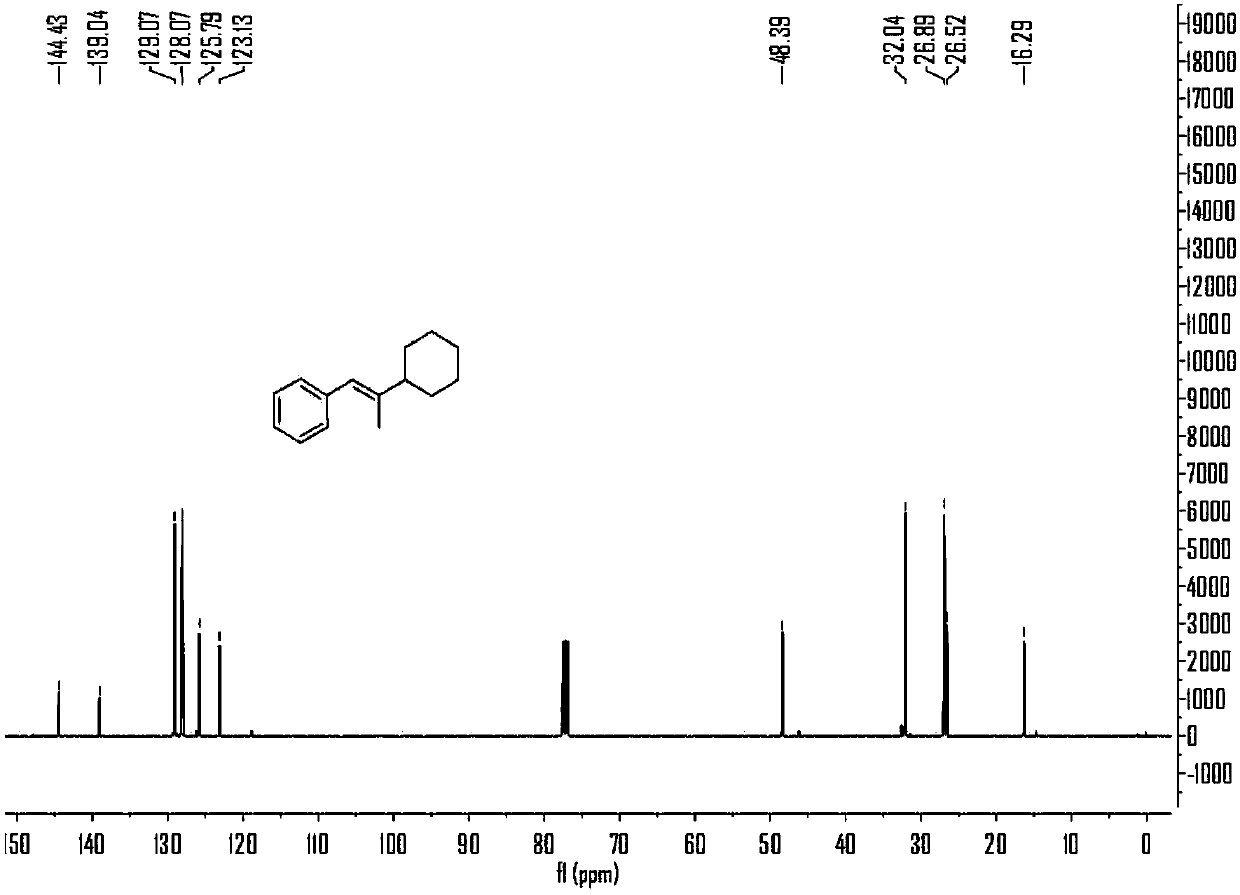
Embodiment 1
[0060] Embodiment 1, the reaction formula of this embodiment is as follows:
[0061]
[0062] (1) Under air, nickel(II) diethylene glycol dimethyl ether complex (12mol%), 4,4'-di-tert-butyl-2,2'-bipyridine (12mol%), carbonic acid Potassium (2.5 eq) was added to a branched, sealed reaction tube containing magnets, which was purged with argon three times. Under argon protection, add 0.7mL N,N-dimethylacetamide to the reaction tube, stir at room temperature for 5 minutes, then add phenylpropyne (2eq) and cyclohexyl iodide (0.2 mmol) and methyldiethoxysilane (3eq) into the reaction solution, plugged the stopcock, and placed in an oil bath at 30°C for 10 hours with stirring.
[0063] (2) Add ethyl acetate to the material obtained in step (1) and mix thoroughly, filter out the solid residue with a short silica gel column, and keep the organic phase.
[0064] (3) Spin dry the solvent in the organic phase obtained in step (2) to obtain a crude product, and then purify the crude p...
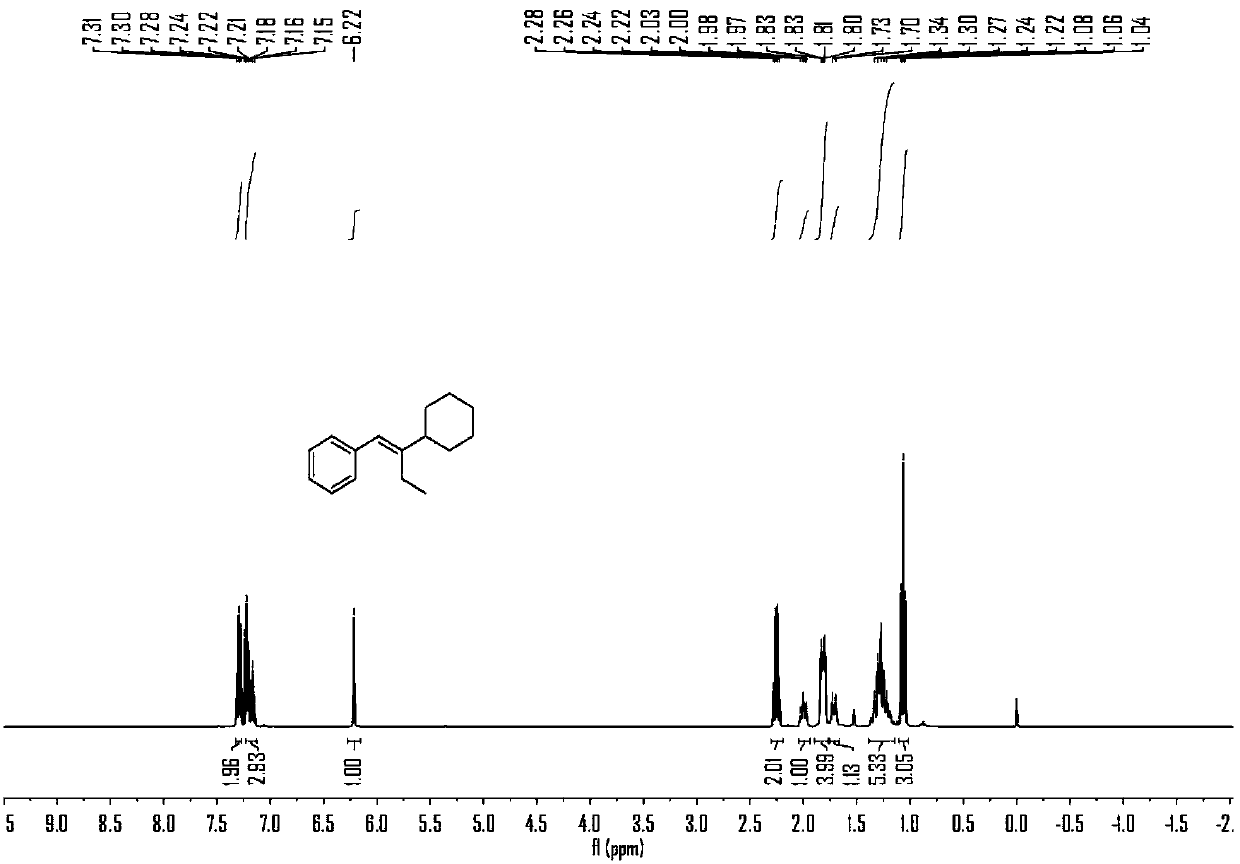
Embodiment 2
[0066] The reaction formula of this embodiment is as follows:
[0067]
[0068] (1) Under air, nickel(II) diethylene glycol dimethyl ether complex (12mol%), 4,4'-di-tert-butyl-2,2'-bipyridine (12mol%), carbonic acid Potassium (2.5 eq) was added to a branched, sealed reaction tube containing magnets, which was purged with argon three times. Under argon protection, add 0.7mL N,N-dimethylacetamide to the reaction tube, stir at room temperature for 5 minutes, then add phenylpropyne (2eq), N-(2- Add iodopropyl)-N-methylaniline (0.2mmol) and methyldiethoxysilane (3eq) into the reaction solution, stopper the stopper, place in an oil bath at 30°C and stir for 10 hours.
[0069] (2) Add ethyl acetate to the material obtained in step (1) and mix thoroughly, filter out the solid residue with a short silica gel column, and keep the organic phase.
[0070] (3) Spin dry the solvent in the organic phase obtained in step (2) to obtain a crude product, and then purify the crude product wi...
Embodiment 3
[0072] The reaction formula of this embodiment is as follows:
[0073]
[0074] (1) Under air, nickel(II) diethylene glycol dimethyl ether complex (12mol%), 4,4'-di-tert-butyl-2,2'-bipyridine (12mol%), carbonic acid Potassium (2.5 eq) was added to a branched, sealed reaction tube containing magnets, which was purged with argon three times. Under argon protection, add 0.7mL N,N-dimethylacetamide to the reaction tube, stir at room temperature for 5 minutes, then add 4-octyne (2eq), 3-iodo- Add 1-p-toluenesulfonylpyrrolidine (0.2mmol) and methyldiethoxysilane (3eq) into the reaction liquid, stopper the stopper, place in an oil bath at 30°C and stir for 10 hours.
[0075] (2) Add ethyl acetate to the material obtained in step (1) and mix thoroughly, filter out the solid residue with a short silica gel column, and keep the organic phase.
[0076] (3) Spin dry the solvent in the organic phase obtained in step (2) to obtain a crude product, and then purify the crude product with...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com